Reports: ND755833-ND7: Sterically-Driven Selectivity in Acyclic Diene Metathesis (ADMET) Polymerization of Asymmetric alpha,omega-Dienes for Sequence-Controlled Polyolefins
Derek L. Patton, PhD, University of Southern Mississippi
Sterically-Driven ADMET Polymerization
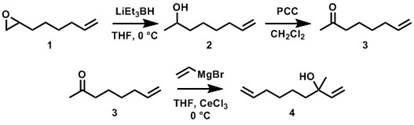
The original objective of this proposal was the investigation of sterically-driven cross metathesis selectivity as an innovative approach for the synthesis of sequence-controlled polyolefins via ADMET polymerization of asymmetric α,ω-diene monomers. Our initial synthetic efforts focused on the preparation of asymmetric α,ω-diene monomers comprising an unhindered Type I α-olefin and a hindered Type III ω-terminal protected tertiary allylic alcohol. Monomer 4 in Figure 1 was successfully synthesized starting from 1,2-epoxy-7-octene (1). However, all attempts employing ADMET polymerization have thus far been unsuccessful and result only in ill-defined oligomeric products. Ongoing efforts are focused on the synthesis of a longer spacer between the α and ω alkenes and the screening of various catalysts.
Figure 1. Synthesis of asymmetric α,ω-diene containing a Type I alkene and a Type II/III tertiary allylic alcohol.
Thiol-ene Chemistry
Support from the current PRF grant has also enabled the exploration of thiol-ene photopolymerization. In the 2015-2016 annual report, we reported a first example of thiol-trifluorovinyl ether (thiol-TFVE) photopolymerization as a facile, cure-on-demand synthetic route to semi-fluorinated polymer networks. This work was published in Macromolecules (DOI: 10.1021/acs.macromol.6b01822). We have extended this approach to other semi-fluorinated thiol-ene resins and fabrication processes that have proven useful for the design and development of oil/water separation membranes.
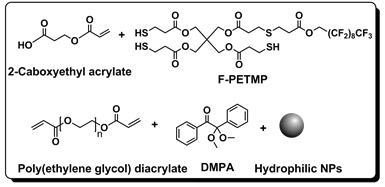
Figure 2. Thiol-ene resin components for photopolymerization.
Here, we report a simple and rapid strategy to fabricate superhydrophilic-superoleophobic surfaces using a hydrophilic and oleophobic thiol-acrylate resin composition. As shown in Figure 2, a thiol-acrylate based hybrid resin was prepared by using a trifunctional thiol with a semi-fluorinated tail (F-PETMP), a hydrophilic alkene (2-carboxyethyl acrylate), and a cross-linker poly(ethyl glycol) diacrylate 400. The photoinitiator employed was 2,2-dimethoxy-2-phenylacetophenone. Hydroxyl-modified silica nanoparticles (SiO2) (Aerosil R380=30, 40, 50, 60 wt.% relative to resin) were employed to achieve a nanoscale surface roughness. Coatings were fabricated via spray deposition. The spray-coating process atomizes the resin solution into a random distribution of micrometer and submicrometer-sized droplets and directs these droplets towards the substrate with air flow. The coating was cured under a UV flood lamp (16 mW/cm2) for 5 min. To simplify the notation for each formulation, we represent the formulations as F-PETMP(x)/SiO2(y), where x and y represents the weight percentage of F-PETMP and SiO2, respectively.
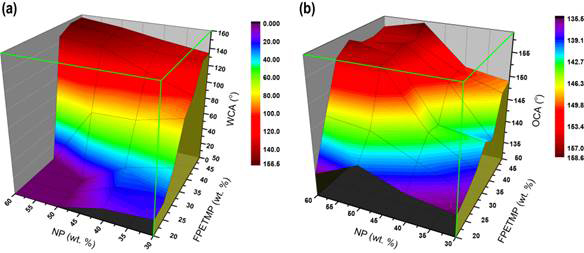
Figure 3. (a) Static water contact angle (WCA) and (b) static oil contact angle (OCA) as a function of thiol-ene resin composition.
We investigated the surface morphology of the F-PETMP(30)/SiO2(50) on glass slide substrate by SEM (data not shown for brevity). Micrometer/sub-micrometer agglomerations were observed on the surface which can be attributed to the spray deposition process and the presence of the silica nanoparticles. Energy dispersive spectroscopy (EDS) analysis was used to explore the surface composition of sample F-PETMP(30)/SiO2(50). The surfaces were primarily composed of F, S, Si, C and O elements. The fluorine content of the cured film obtained from EDS was 8.78±0.13 wt.% while the content calculated from the bulk resin composition for F-PETMP(30)/SiO2(50) was 5.33 wt.%. The higher F content result indicates the migration and segregation of fluorine-carbon chain from the bulk to the surface, which provides the basis for the superoleophobicity of the surface.
Figure 3 shows the static water contact angle (WCA) and oil contact angle (OCA) as a function SiO2 nanoparticle (NP) loading level and F-PETMP content. For brevity, we focus on the resin composition that provided superhydrophilic and superoleophobic wetting behavior. F-PETMP(30)/SiO2(50) showed a minimum WCA value of < 5° and a maximum OCA value of 155.6±1° with oil sliding angle of 10±1° which indicated superhydrophilic/superoleophobic wetting behavior. The F-PETMP(0) SiO2(50) resin was used to conduct an oil/water separation study. We assumed that substrates also influence the performance of separation, so coatings were prepared on a series of substrates including stainless steel mesh (200 mesh size), cotton cloth and nylon filter membranes (0.8 and 0.45 μm pore size) to evaluate their oil/water separation efficiency. Figure 4 show the typical surface morphology of porous nylon membrane with 0.8 μm (a) and 0.45 μm (b) pore size. Figure 4c shows the top view SEM image of sample F-PETMP(30)/SiO2(50) on 0.45 μm pore size nylon membrane, which has a similar dual-scale hierarchical structure as discussed previously. The cross-section of the coated sample was studied by both TEM (Figure 4d) and SEM (Figure 4e-f). As shown, the coating covers the membrane, but has not blocked all the pores of the membrane enabling the transport of liquid for separation processes.
Figure 4. (a) SEM of nylon membrane with 0.8 μm pore size and (b) 0.45 μm pore size. (c) Top image of sample F-PETMP(30)/SiO2(50) on 0.45 μm pore size nylon membrane; (d) TEM of cross-section view of F-PETMP(30)/SiO2(50) spray coated nylon membrane; (e) and (f) SEM cross-section of the spray coated nylon membrane.
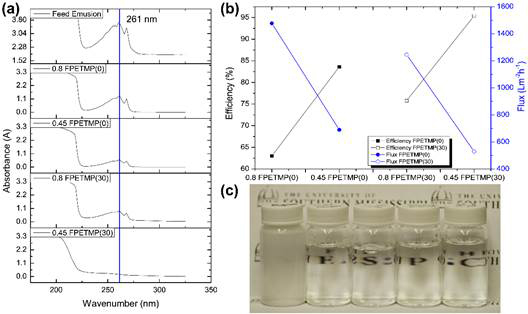
Figure 5. (a) UV-VIS of the toluene-in-water emulsion before and after separation; (b) separation efficiency and flux of a serial sample in terms of the oil rejection and permeate flux; (c) Photograph and optical images of feed emulsion and filtrate separated by 0.45 F sample.
The separation performance of the coated nylon membranes was investigated using a simple set up in which the spray coated membrane was fixed between the upper part and the bottom part of the funnel. The receiving bottle was applied with 30 KPa pressure. The filtered emulsions (toluene-in-water emulsion with SDS as surfactant) were analyzed with UV-VIS spectrometry using the absorbance peak of toluene at 261 nm (Figure 5a). The separation efficiency was determined per literature. The calculated separation efficiency and water flux results for PETMP(0)/SiO2(50) and F-PETMP(30)/SiO2(50) are shown in Figure 5b. For both coatings, decreasing the pore size of the membrane resulted in improved efficiency and decreased flux. For each pore size, F-PETMP(30) shows a higher efficiency and lower flux compared to F-PETMP(0); this behavior can be attributed to the additional fluorine content and increased rejection of the oil droplets. The purity of the recovered water for 0.45 F-PETMP(30) was 95.4% which indicates a good efficiency, while the flux was 529.31 Lm-2h-1 which is still higher than the conventional ultrafiltration membranes.