Reports: ND256972-ND2: Investigating the Fossil Record of Early Eukaryotes Using Sterane-Specific Antibodies
Jake Bailey, PhD, University of Minnesota
Overview: Where, when, and how did the eukaryotic cell originate? What sorts of ecologies and physiologies did early eukaryotes have? What selective processes led to the origin and diversification of eukaryotes? These are some of the questions we hope to address through the developing, testing and application of sterane-specific antibodies. We have made some substantial progress in several of our specific aims, including evaluating the specificity of anti-stigmastane antibodies, developing new antibodies, and applying antibodies to biomarker detection in natural samples.
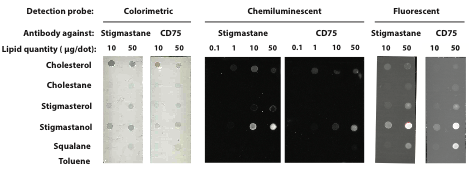
Evaluating sterane-antibody specificity: Our first proposed aim was to evaluate the anti-sterane antibodies against lipid standards to ensure that they are sterane-specific and do not cross-react with other sedimentary hydrocarbons. We began working with anti-stigmastane monoclonal antibodies that we produced several years ago. Our preliminary immunoblot results show that the antibodies do indeed appear to bind somewhat specifically to steranes using a colorimetric- and a chemiluminescence-based assay (below, left and middle panels), while a fluorescence-based assay (right) does not show specificity compared to the negative control (anti-CD75). The reason for this is not yet entirely clear, but these preliminary results are intriguing and show that steranes can be targeted using a high-sensitive chemiluminescence method.
Developing new antibodies: In addition to testing our previously produced anti-stigmastane antibodies, we tried obtaining more antibodies from the original antibody-secreting cells stored at the University of Southern California. Unfortunately, the cell lines were inadvertently destroyed. While we still possess anti-stigmastane antibodies that we can use for this project to evaluate our approaches, the only long-term source of sterane-specific antibodies is now inaccessible. As such, we are working with the USC antibody production lab to generate new anti-sterane monoclonal antibodies. We recently produced liposomes containing cholestane, which will be the antigenic substrate for the antibody-production project currently in progress. Within a few weeks, we will begin testing antibodies for sterane reactivity using enzyme-linked immunosorbant assay. The clones showing reactivity to steranes, but not to a mixture of other non-sterane hydrocarbons, will be selected for further study. We also plan to deposit the hybridoma cells with the American Type Culture Collection to avoid destroying or losing the the cell lines, so that other researchers will also have access to them. The production of new sterane antibodies was part of our original proposed research, so by producing antibodies against cholestane, we are not only replacing the lost anti-stigmastane antibodies, but also making antibodies to target different steranes.
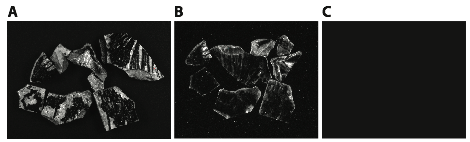
Evaluating antibody binding in natural samples: Before using anti-sterane antibodies to detect steranes in fossils that are not definitively known to contain sterols, such as Chuaria, we should first test them on fossils that either should contain sterols because we are confident they represent eukaryotes, or we should test the antibodies on microfossils that are known to contain sterols by other methods. We also need to optimize the reporter system that we plan to use for visualizing the bound antibodies in situ. Our initial efforts to apply antibody-based detection of biomarkers in natural samples involve two approaches. First, we used anti-squalene antibodies to attempt detecting squalane in fish fossils from the Eocene Green River Formation. The anti-squalane antibody was developed by a different laboratory for a different purpose. We are using these anti-squalane antibodies to develop our in situ staining procedures as well as showing the antibody potential as an organic geochemistry tool (Medina et al. in review). Below are some preliminary results showing fish fossil fragments exposed to anti-squalane antibodies under (A) epi-white light; (B) a chemiluminescence substrate with no illumination; and (C) a negative control of the fossil with the chemiluminescence substrate but without anti-squalane antibodies. The chemiluminescence signal (B) shows antibody binding to squalane or other similar hydrocarbons in the sediment matrix surrounding the fish fossil. The preliminary results illustrate the potential for using a chemiluminescence reporting system for imaging the antibody-biomarker distribution in natural samples.
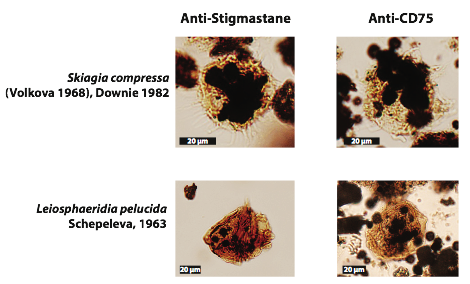
In addition to working with the squalane antibodies on fish fossils, our second approach is applying our anti-sterane antibodies to natural samples. There are only one or two populations of microfossils that have been shown to contain steranes by GC-MS. The acritarch assemblage from the Lower Cambrian Lükati Formation in Estonia is one such rare assemblage. We have obtained samples from the Lükati Formation and dissolved them using hydrofluoric acid to isolate the acritarchs for use in our antibody-staining experiments. We used fluorescence microscopy to evaluate the autofluorescence characteristics of the acritarchs. Because some acritarchs autofluoresce, we used a colorimetric approach (based on CN/DAB substrate) to visualize the bound antibody by a brown precipitate. Shown below are two representative fossils from the Lükati Formation incubated with the anti-stigmastane antibodies (left) and the isotype control anti-CD75 (right). Color precipitation is observed in the lower left panel, whereas its control and the upper panels do not show obvious colorimetric substrate precipitation. We are currently working on developing a more sensitive, low-background, reporter method to detect the antibody interaction by a chemiluminescent substrate.
These results, while still preliminary, do suggest that antibodies raised against biomarker compounds can be fairly structurally specific, and that they can be coupled to various reporter substrates to detect their binding in natural samples.
Participants: The work was primarily performed by my Ph.D. student, Mr. Fernando Medina-Ferrer who is using this project as the basis of his Ph.D. thesis. We also hosted a Master's intern, Ms. Cécile Bidaud, from The Institute de Physique du Globe de Paris. Ms. Bidaud was mentored by Mr. Medina-Ferrer and contributed to some of the results described here.
Dissemination of research results: Mr. Medina-Ferrer presented some of his research results from this project at the 3rd Annual ESCI Student Research Symposium, April 14 (2017), University of Minnesota, and at the 1st Geobiology Society Conference, June 11-14 (2017) in Banff, Canada. Ms. Bidaud also presented some of these results as part of her successful thesis defense on August 31, 2017 at the IPGP, Paris, France.