Reports: DNI556991-DNI5: Computational Design of Growth Inhibitors for Tailoring Zeolite Catalysts
Jeremy Palmer, PhD, University of Houston
This ACS PRF grant supported two research thrusts in our lab related to using advanced molecular simulation techniques to understand how small organic molecules can be used to tailor zeolite catalysts. The principal focus of our investigation is on understanding how zeolite growth inhibitors (ZGIs) selectively adsorb to different crystal faces to modulate anisotropic zeolite growth rates during synthesis. Recent experiments show that ZGIs can be used to produce thin zeolite crystals, which exhibit enhanced catalytic lifetimes and performance characteristics. Additionally, we have also expanded the scope of our initial investigation to understand how organic structure directing agents (OSDAs) can be used to stabilize different zeolite structures and control polymorph selection during zeolite synthesis. Below, we describe major accomplishments over the past year.
(1) Molecular scale hydrophobicity of zeolite surfaces
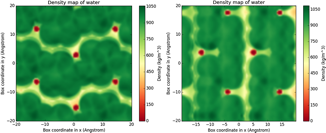
Over this project year, we have used molecular simulation to investigate the structure of aqueous solvents near the different crystallographic surfaces of silicalite-1. Experiments suggest that the efficacy of ZGIs correlates with their molecular hydrophobicity. Consequently, we posit that that ZGI binding may be driven by hydrophobic association and influenced by solvent structure near the zeolite surface. To investigate this hypothesis, we have used molecular dynamics simulations to probe the behavior of water near the surface of silicalite-1 (Figure 1). Local hydrophobicity of the surface is characterized by examining water density distributions and fluctuations, which have been shown to correlate with traditional macroscopic metrics of hydrophobicity such as contact angle measurements. At the microscopic scale, density fluctuations allow us to identify hydrophobic and hydrophilic patches on the zeolite surfaces that may serve as favorable adsorption sites for ZGIs. The crystallographic faces of silicalite-1 are predominately hydrophilic due the presence of exposed silanol groups on the surface, which form strong hydrogen bonds with water. Our analysis of surface hydrophobicity maps reveals, however, that there are hydrophobic crevasses on the surfaces where hydrophobic ZGIs may selectively bind. The next step in our investigation will be to use molecular simulation to study how different ZGIs bind to these hydrophobic regions. Specifically, we will use enhanced sampling methods to compute binding free energies for hydrophilic and hydrophobic ZGIs. The binding free energies from simulation will be compared with experiment to correlate binding strength with ZGI efficacy.
Figure 1. Water density maps for the 010 (left) and 100 (middle) surfaces of silicalite-1 computed with molecular dynamics.
(2) Cetyl trimethylammonium as an OSDA for MFI
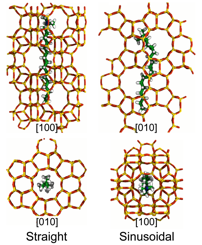
We have also used molecular simulation to investigate how OSDAs stabilize the MFI framework. Tetrapropylammonium (TPA) is the most commonly used OSDA in MFI synthesis. Recent experiments show, however, that the surfactant cetyl trimethylammonium (CTA) can also be used as an OSDA in MFI synthesis. To understand how CTA stabilizes MFI, we performed molecular dynamics simulations to compute the stabilization enthalpy for different configurations of CTA within the MFI framework. We find that there are at least two distinct conformations accessible to CTA that results in stabilization of MFI. In the first conformation, CTA occupies a straight channel with its trimethylammonium head group extending into one of MFI's large pores. In the second conformation, CTA's backbone bends to allow it to occupy a sinusoidal channel. While both conformations are favorable, the former leads to greater framework stability and thus will be energetically preferred. Nevertheless, the similar magnitude of the stabilization energies suggest that CTA may be occluded in both conformations during zeolite synthesis. Further, the stabilizing energies are comparable to those observed for TPA. This finding suggest that chain-like surfactants can be as effective in stabilizing MFI as traditional OSDAs such as TPA, and it provides a molecular level explanation for how CTA promotes MFI formation.
Figure 2. Snapshots of CTA's conformations. In the most stable conformation (bottom left), CTA's major axis is aligned with MFI's straight channel and its head group protrudes into the void space where the straight and sinusoidal channels intersect. The MFI framework is also stabilized when CTA occupies the sinusoidal channel (bottom right).
(3) Cooperative OSDAs for zeolite MOR
We have also used simulation to investigate synergistic effects between OSDAs in the synthesis of MOR. Recent experiments show that MOR framework zeolites can be synthesized using a combination small alcohols and ammonia compounds as OSDAs. Remarkably, the MOR framework does not form when either type of OSDA is used separately. This observation suggests that the two OSDAs function in a cooperative manner to stabilize MOR. Similar cooperativity has not been previous reported in the literature. Preliminary results from our simulations suggest that the two OSDAs are occluded in different regions of MOR such that the void spaces of the framework are completely occupied with organics. By contrast, when either OSDA is used separately, portions of the void space remain unoccupied due to steric hindrance. Hence, the combination of both OSDAs enhances stability of MOR. This intriguing finding opens the possibility of using mixtures of OSDAs to stabilize zeolites that currently challenging to synthesize using existing single OSDA protocols.