Reports: UR654065-UR6: Between Oil and Water: Computational Modeling of Molecular Structure and Interactions at Surfactant Modified Hydrophobic Interfaces
Kevin E. Johnson, Ph.D., Pacific University
Professional career impacts:
Kjersti Chippendale, an undergraduate student who was supported by PRF funds in Summer 2016, graduated spring 2017 with a chemistry major. She presented a poster at the Spring 2017 ACS national. Ms. Chippendale plans to pursue a career in chemical education.
Four undergraduate students worked Summer 2017 in the Johnson lab, two received support from the PRF grant and two from Pacific University endowment funds. The PRF-funded students were: Logan Naegeli, a senior bioinformatics major, who worked on coding custom software for analysis of molecular dynamics (MD) trajectory data, and Todd Martin, a senior chemistry major, who prepared, executed, and analyzed MD simulations of water/organic interfaces with surfactants and divalent cations. Mr. Martin is continuing to work in this project for his senior capstone project and he will attend the spring 2018 ACS meeting. He is planning on pursuing graduate studies in the chemical sciences.
The two students who received summer salary from Pacific University through endowed funds supporting research were: Kory Melton, a senior bioinformatics major planning on graduate studies in computational biology, and Nathaniel Olowo, a senior chemistry major planning on studying medicine after graduation. Both students worked in a molecular dynamics study of small-molecule stabilizing interactions with enzymes to inhibit thermal denaturing. (The student wages and equipment used on this project were funded from sources other than PRF. However, they were part of the four-student research team this past summer supporting each other’s research work.) Both Mr. Olowo and Mr. Melton will continue their work for their respective senior capstones. Mr. Olowo will present a poster on his work at the Spring 2018 ACS national meeting.
For the PI, Professor Johnson, PRF funding has provided the opportunity to expand the computational methods used in his lab to include molecular dynamics as applied to surfactant and biological systems.
Scientific progress:
Below are initial research outcomes from modeling the effects of aqueous divalent cations on the interface structure of dodeconate surfactants following the work in the Richmond lab at the University of Oregon. (Robertson, E. J., Beaman, D. K., & Richmond, G. L. (2013). Designated Drivers: The Differing Roles of Divalent Metal Ions in Surfactant Adsorption at the Oil–Water Interface. Langmuir, 29(50), 15511–15520.
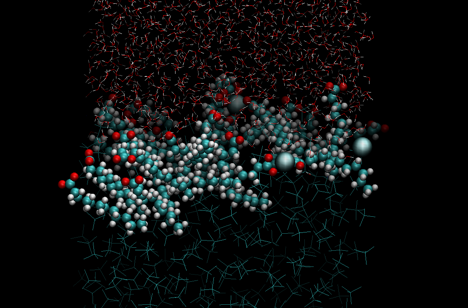
We simulated a water/carbon tetrachloride interface with a saturated interfacial concentration of dodeconate and 0.10M aqueous calcium chloride via a canonical ensemble in a periodic box. A representation of the equilibrated structure is illustrated in Figure 1.
Figure 1: Visualization of the simulation interface after 20ns at 300K. CCl4 is in the lower portion of the image and the aqueous layer on top both represented as transparent lines. The surfactant and Ca2+ ions are represented by van der Waals spheres.
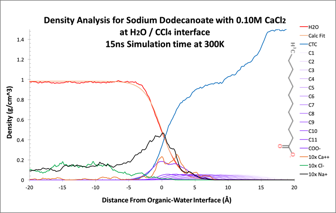
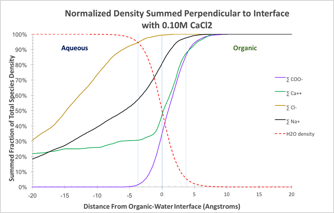
Our analysis averages density by species as a function of distance from the interface as defined by the water layer. Figure 2a illustrates the density plot. By summing the density by slice starting in the aqueous layer through to the organic layer, we can see how each species interacts at the interface as illustrated in Figure 2b.
A
B
Figure 2: Density analysis by species. A Average density calculated by slice parallel to the interface. B The normalized sum of density starting in the aqueous layer.
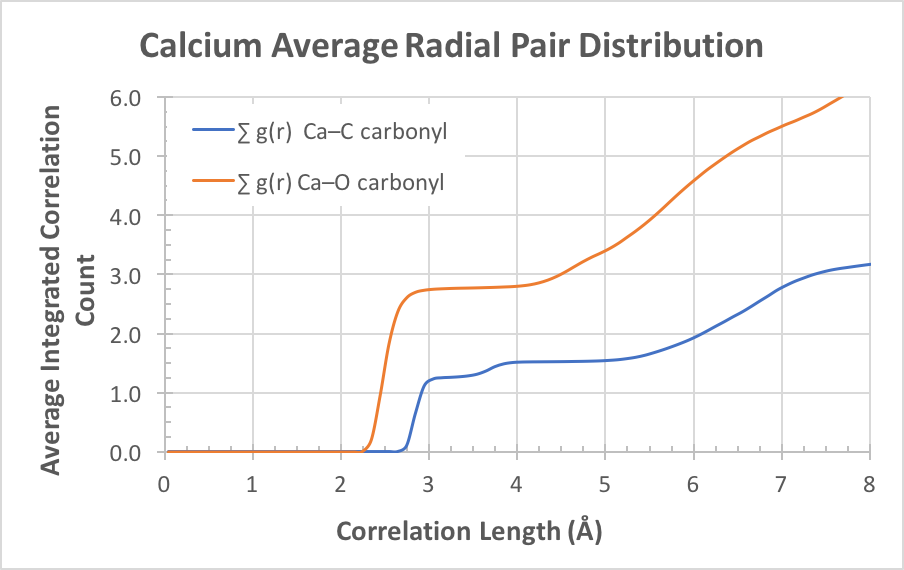
The density sum shows a significant excess concentration of sodium and calcium at the interface. In this simulation, three of the four calcium ions present in the simulation were in the interface layer. The calcium ion also appears to track the dodeconate head group in interface, suggesting a close attractive association. Figure 3 illustrated the integrated radial distribution analysis of calcium ions with the carbonyl oxygens and carbons. The correlation indicates that on average the calcium ions in the simulation are closely associated with 2.75 carbonyl oxygens at a separation of 2.9Å. At greater separation, the distribution of carbonyl carbon atoms increases sharply from zero to a value of 1.25 at 3.2Å, increasing to 1.50 at 3.95Å. This indicates that 5 of 6 carbonyl associations with calcium ion are through both oxygen atoms, and the remainder are single oxygen associations. Closer visual inspection of the simulation graphics indicates that a single calcium ion can attract as many as 3 pairs of carbonyl oxygens plus one single carbonyl oxygen. We conclude that calcium ions (2+) are pulled from the aqueous layer via charge-charge attractions with the carboxylic head groups (1-) of the surfactant, and form strong close ionic associations.
Figure 3: Radial pair distribution functions summed to illustrate bonding count and type.
This project will continue with simulations for 0.050M and 1.0M CaCl2 plus simulations with other divalent cations.