Reports: ND1057060-ND10: Mixing Oil and Water by Ionic Liquids: Stability Maps of Emerging Fluids in Oil Recovery
Cecilia Leal, PhD, University of Illinois, Urbana-Champaign
PROJECT GOALS: In this project we investigate the ability of Ionic Liquids (IL), instead of surfactants, to stabilize mixtures of oil and water. This fundamental science research can potentially impact the search for new fluids in enhanced oil recovery. The project is organized under two specific aims:
Specific Aim 1. The Oil/Water/IL phase map: investigation of the ternary phase diagram of Oil/Water/ILs using high molecular weight oils and two distinct ionic liquids: more hydrophilic ones and more hydrophobic ones.
Specific Aim 2. Ternary solutions under confinement: Investigate the phase behavior of Oil/Water/IL when confined between solid surfaces. ILs can display peculiar behavior under confinement. In this aim we will investigate the structures of the ternary mixtures, in particular the single-phase regions of Oil/Water/IL (that possibly resemble surfactant-based microemulsions) under confinement. This is important because real oil reservoirs are confining environments.
MAJOR ACCOMPLISHMENTS: During this reporting period we pursued a few objectives of Specific Aim 1 as well as Specific Aim 2. The main tools employed were: i) Small Angle X-ray Scattering (SAXS), ii) visual inspection of ternary mixtures, iii) microscopy, and iv) Surface Force Apparatus (SFA).
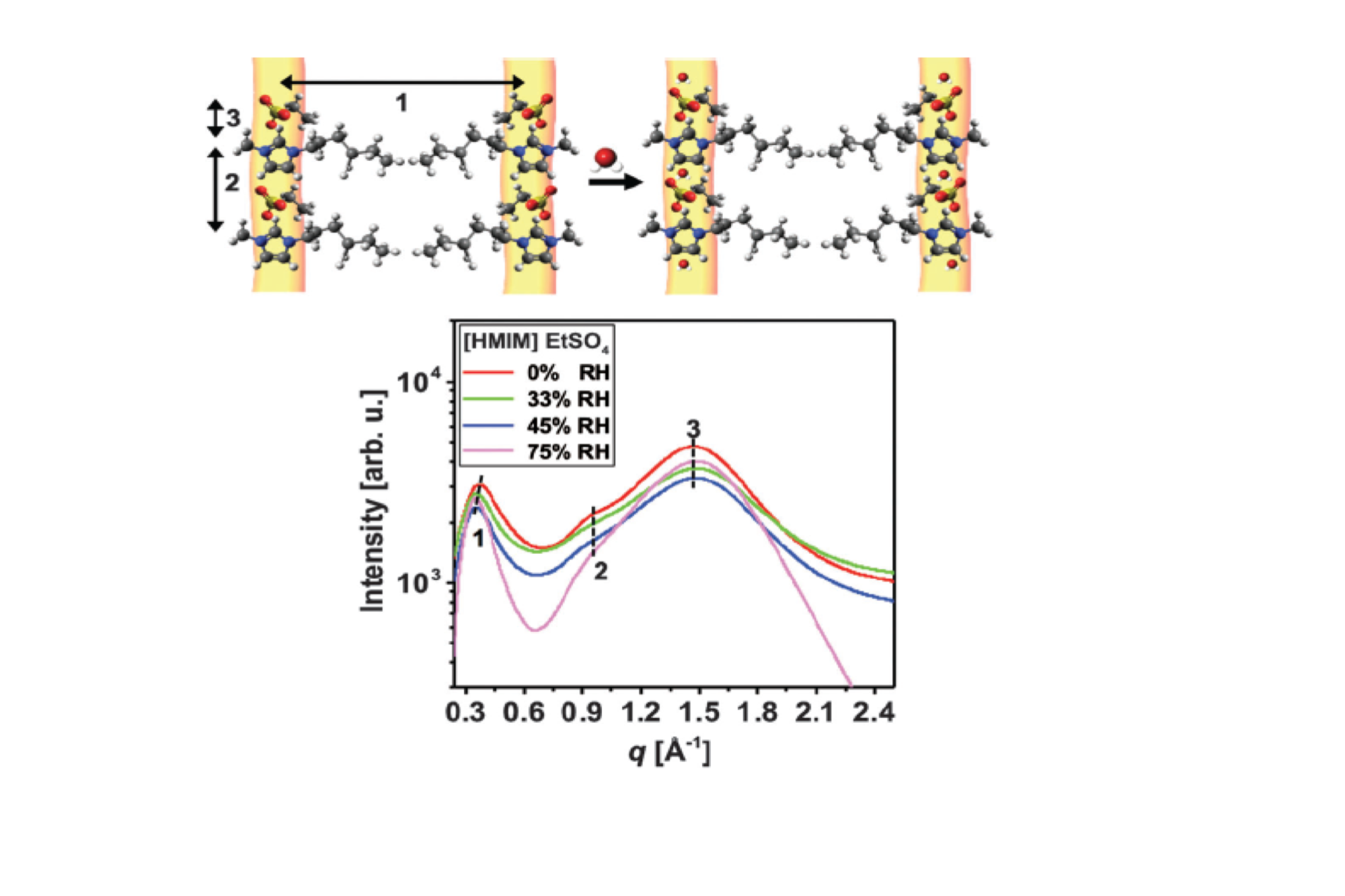
Significant results and findings 1. We firstly investigated binary (water + IL) mixtures before inspecting the ternary phase diagram. We studied the effect of environmental humidity on an hygroscopic IL ([HMIM] EtSO4) with and without confinement. SAXS results reveal a layered structure of ILs that expands upon water uptake. When the water-containing [HMIM] EtSO4 is nanoconfined between two mica surfaces, no long-range order is detected, in contrast to the results obtained for the dry IL, which demonstrates that the presence of water can prevent the liquid-to-solid transformation of this IL. The SAXS results are depicted in Figure 1 below.
Figure 1: SAXS for [HMIM] EtSO4 exposed to dry (red line), 33% RH (green line), 45% RH (blue line), and 75% RH (pink line). Peak at the q ranges of 0.3 to 1.8 1/Å (marked by 1, 2, 3) correspond to ordering of the ILs as depicted in schematics of the top figure. As water is added to the IL there is a modest q shift of only the first peak corresponding to a swelling of the IL.
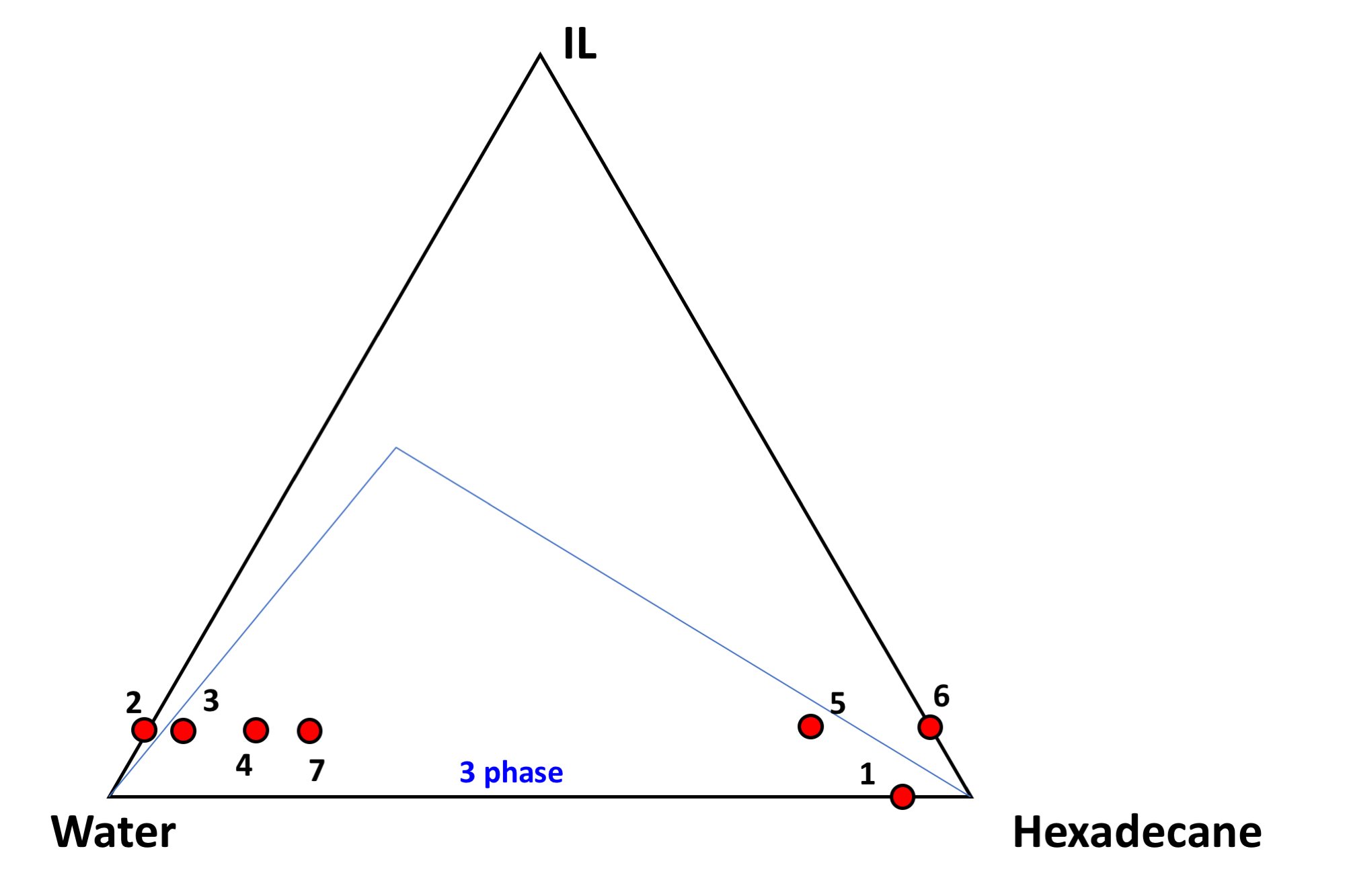
Significant results and findings 2. Ternary mixtures of Water, Hexadecane, and a IL with equal affinity for water and oil (P66614Cl) were tested with respect to the ternary phase diagram. A number of different phase regions were identified visually. Importantly, a few samples displayed a three-phase region consistent with water and oil separated by an isotropic fluid which is a thermodynamically stable microemulsion. The ternary phase diagram is depicted below in Figure 2.
Figure 2: Ternary phase diagram showing several samples (4, 7, and 5) where a microemulsion between water and oil exists which is stabilized by an IL instead of a classical surfactant system.
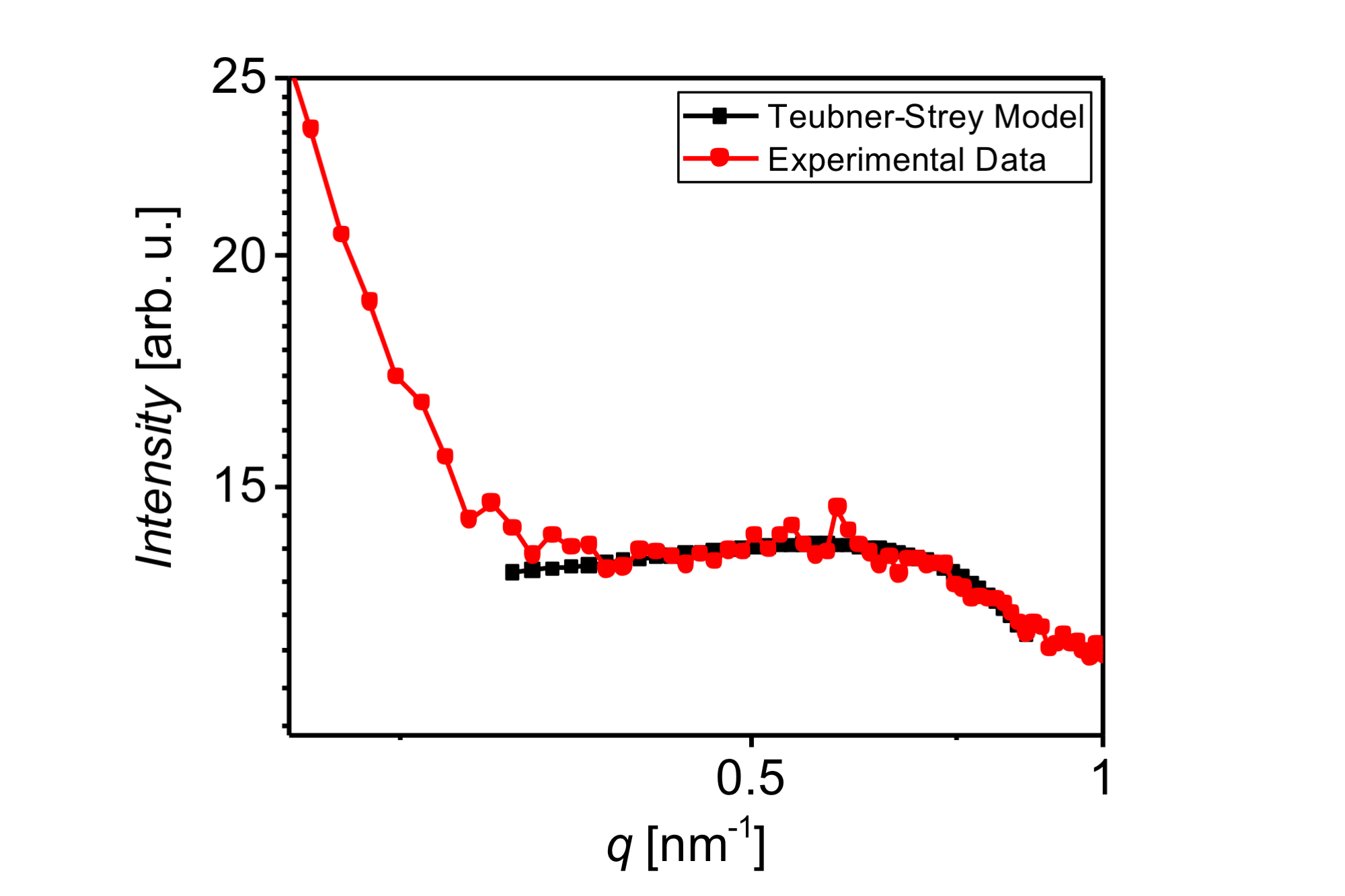
Significant results and findings 3. The microemulsion samples were investigated by SAXS and found to be constituted of droplets of ca 7 nm by fitting a Teubner-Strey model. The correlation length is 2 nm indicating that this is a dense microemulsion system. The SAXS results are shown in Figure 3 below. To our knowledge this is the first structural finding an IL that is able to mix oil and water in an isotropic solution.
Figure 3: SAXS results for sample #7. The SAXS profile is typical of isotropic fluids with droplet like microemulsions. The Teubner-Strey model yields a domain size of about 7 nm and a correlation length of 2 nm.
Significant results and findings 4. Relevant microemulsion samples were inspected under confinement between mica sheets by SFA. It was found that in the microemulsion, a long-range force is observed between 15 and 40 nm, which is tentatively attributed to the osmotic effects of concentrating the microemulsions under confinement. It was also found that the droplets deform at moderate loads. Such a long-range force was not observed in the oil-phase reference samples. Film thickness transitions were observed at high loads, only in microemulsion samples. Based on a statistical analysis over 60 force curves measured on 4 different mica pairs, the film thickness transition closer to the hard wall is measured at 0.4 nm, with the farther one being at 1.6 nm. The two transitions are attributed to two well-organized layers of water and microemulsions being squeezed out from the region of contact.
FUTURE PLANS: In the remainder time of the proposal we will continue to use a combination of SAXS and ternary phase diagram determination of a few IL to determine a vast number of systems in which microemulsions are observed. We anticipate that structured microemulsions deviating from droplets (such as bicontinuous type) may exist for some systems. For the samples that are stabilized as microemulsions we will evaluate the effect of confinement using SFA and mica sheets. Finally, we will inspect how added salinity affects the behavior of the microemulsions with and without confinement.