Reports: UR1055690-UR10: Understanding the Behavior of Guest Molecules within Zeolite Materials
Jennifer S. Holt, PhD, Valparaiso University
Research Overview
We continue to make progress on the goals set out in our ACS PRF grant, which aims to understand and optimize the behavior of dye molecules within zeolite channels. Host-guest systems are often explored to create a novel property or unique application, but the fundamental understanding of the factors affecting this behavior is not often explored. This grant focuses on comparing the behavior of a dye molecule under a wide variety of conditions in order to better understanding how zeolite channels can be used to create new materials with unique properties. Through the academic year, three undergraduate students were involved in this project, and they were able to verify results obtained during year one of the grant, as well as make progress on new analyses. These students continued to optimize our crystal growth of Zeolite L and the dye insertion process, and we used various characterization methods to determine the factors that lead to maximum dye loading of the zeolite crystallites.
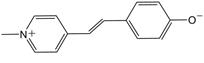
More specifically, the dye molecule of interest in this project is called Brooker’s merocyanine (shown in Figure 1). It can be both protonated and isomerized, leading to a variety of structural forms that can be inserted into the channels of Zeolite L. Zeolite L was chosen because of its simple one-dimensional channel structure that was the appropriate dimensions and chemical composition (intermediate Si:Al ratios) to incorporate conjugated dye molecules. Over the past year, we focused on the insertion of both the protonated and deprotonated forms of Brooker’s merocyanine from an aqueous solution. We found that the protonated dye was much more likely to interact with the Zeolite L crystallites as a result of cation exchange within the channels than the deprotonated form. We characterized the number of dye molecules that were adsorbed per gram of zeolite, and we found that the results indicated more molecules were adsorbed than could be accounted for by simple surface coverage, so there is likely dye insertion into the channels. BET analysis will be performed to confirm this analysis by comparing the pore volume of the original and dye loaded zeolites. We have also begun to develop strategies to study how the morphology of the Zeolite L crystallites may affect dye loading by altering the length and number of channels in the crystallites. We will pursue these studies in the upcoming year in addition to further exploring dye loading characteristics and the possibility of organization within the channels by measuring nonlinear optical properties of these materials.
Figure 1: Structure of Brooker’s merocyanine.
Although we were able to make progress over the course of the academic year, our most significant time for research progress over the summer was stunted due to the completion and move to a new science building at Valparaiso University. The new building is almost entirely wet labs, and it will house all of the Chemistry Department and some of the Biology Department. One of the main objectives of the new 55,000-square foot building is to expand and improve the research facilities, including more and improved space for research projects, more instrumentation, and better infrastructure compared to our old labs. In addition, we had to vacate our previous labs to begin renovation of those spaces for the remaining Biology and Physics faculty. Unfortunately, due to the actual move and delays in occupying the new space, very little progress was achieved over the summer. However, we are now fully operational and we are continuing to investigate these dye-zeolite materials in a wonderful lab space.
Impact on Undergraduate Students and the PI
Three undergraduate students were involved in this research project over the past year. Two students continued their work from the previous summer, and they were able to address some questions that remained unanswered during their summer research. One additional student joined the project as a sophomore, and she worked with one of the experienced students to understand the techniques and background in order to take over the project as they finished up their research experience. Each of these students presented their results at various poster and oral presentations on campus over the past year to a variety of audiences, ranging from a chemistry seminar for 50 students and faculty to a campus-wide research symposium. Their experience as part of this research program led to further opportunities off-campus over this past summer, where one student was able to obtain an industrial internship over the summer, and the other student was able to shadow a pharmacist in order to prepare her for pharmacy school. This grant has also allowed me to purchase supplies necessary for this work, as we set up my lab in the new building.