Reports: DNI556197-DNI5: Direct Conversion of Methane to Valuable Chemicals Using Single-Atom Catalysis
Yu Lei, PhD, University of Alabama, Huntsville
Major Goals and Objectives
This ACS PRF sponsored project builds around rational design of highly effective iron-oxide based catalysts for non-oxidative methane dehydroaromatization (MDA) to valuable chemical products that will solve such problems as efficient utilization of natural and oi-associated gas, and environmental protection. The major goal of this PRF project is to create “nanobowl” structured iron-based catalysts for non-oxidative MDA reaction. Specifically, our research aims at understanding:
· Catalyst synthesis for optimizing the thermal stabilize using atomic layer deposition (ALD)
· The active site(s) for non-oxidative MDA created by the iron oxide and support interfaces
· The selectivity to ethylene and aromatics controlled by the coordination and the accessibility of the active sites.
Achievements
With the support of PRF grant, great efforts have been taken to develop synthesis strategy using ALD to design and synthesize advanced catalysts, characterize these catalysts using in-situ characterization techniques at synchrotron source, and design and kinetic test of the catalysts.
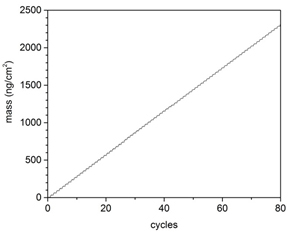
We can deposit several metal oxides with different coverage on the support to study the coverage effect. The ALD timing sequences can be expressed as t1-t2-t3-t4, where t1 is the exposure time for the first precursor, t2 is the purge time following the first exposure, t3 is the exposure time for the second precursor, t4 is the purge time following the exposure to the second precursor, and all units are given in seconds. We have established a recipe for preparing TiO2 ALD recipe using using alternating exposure of TiCl4 and H2O at 100 °C as shown in Figure 1 with 1-5-1-5 time sequence. FeOx ALD was realized using alternating exposure of Fe(Cp)2 and ozone with 2-10-2-10 time sequence. A growth rate of 1.2 Å per ALD cycle has been established using this method, consistent with the literature. The results have been obtained using both in-situ quartz crystal microbalance (QCM) housed in the ALD chamber and further confirmed using spectroscopic ellipsometer. A much longer exposure time was established for coating metal oxides using ALD on high surface area supports. We have also established various metal oxide ALD processes as possible materials for non-oxidative MDA, including Al2O3, SiO2, ZrO2, etc.
ALD was applied to modify the external surface of the MFI and MWW zeolites. In this effort, the external surfaces of zeolites were coated with SiO2 and Al2O3 using ALD, respectively. As a result, the external surfaces of zeolites represent well-controlled Si- and Al-termination. The presence of the various termination effective altered the surface acidity and reaction selectivity.
|
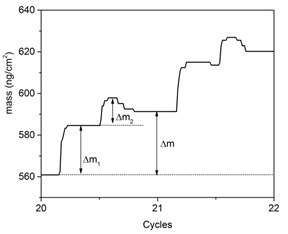
Figure 1. In situ QCM studies of TiO2 ALD using TiCl4 and H2O as precursors at 100 °C. δm1 and δm2 represent the mass gain during TiCl4 and H2O exposure, respectively. The growth rate of TiO2 is ca. 0.67 Å per ALD cycle.
|
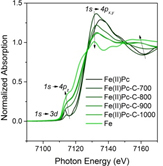
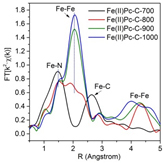
We have also studied the effects of the use of different deposition precursors such as ferrocene (Fe(Cp)2) and iron (II) phthalocyanine (Fe(II) Pc). We can measure the iron-based catalysts for non-oxidative MDA under various temperature and chemical environment. As demonstrated in Figure 2, synchrotron-based X-ray absorption spectroscopy (XAS) is able to precisely resolve the oxidation state and nearest neighbors of the Fe atoms. With increasing annealing temperature, the XANES spectra gradually resembled that of the metallic Fe, indicating a slow reduction of the Fe species oxidation state. After annealing at 973 K, the disappearance of Fe-C bond in EXAFS spectra indicated the loss of the –Pc ligand and possible formation of iron nanoparticles, leading to emerging Fe-Fe bonds at about 2 Å (phase uncorrected). Detailed EXAFS data fitting showed that the Fe-Fe coordination number increased from 2.1 after annealing at 800°C to 4.4 after annealing at 1273 K. Fe-O, Fe-C, and Fe-Fe bonds could appear during the non-oxidative MDA reaction conditions, depending on the reaction temperature and reactant composition. Besides iron-based catalytic materials, we have also characterized lead substituted hydroxyapatite (Pb-HAP) which was investigated as active catalysts for oxidative coupling of methane (OCM) with our collaborator Dongxia Liu’s group.
|
Figure 2. Fe K-edge (A) XANES and (B) Fourier transfer EXAFS of model compound Fe(II)Pc annealing at elevating temperatures. Data were taken at APS.
|
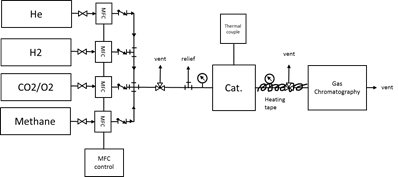
A catalyst kinetic testing system has been designed and assembled. The sketch of the system is illustrated in Figure 3. The system will allow us to investigate non-oxidative MDA as well as oxidative methane coupling and light alkanes oxidative dehydrogenation (ODH).
|
Figure 3. A scheme of catalyst testing system.
|