Reports: UR555886-UR5: In Situ Generated Two Dimensional Metal and Bimetallic Nanoparticle Catalysts
Krisanu Bandyopadhyay, University of Michigan, Dearborn
The objective of the present research is to generate nanoparticle (NP) assemblies in situ inside a suitable template on a solid surface to avoid a number of sequential steps and pre-synthesized nanoparticle in solution. Moreover, the template will essentially act as a reaction chamber that provides a scaffold for immobilization of specific metal ions, prevent aggregation, and further act as capping agent to control the growth of the desired nanoparticle structure. In this present research project, we propose to generate in situ 2D assemblies of metallic (Au), and bimetallic (Au-Pd) nanoparticles inside different silane monolayer templates on silicon and indium tin oxide (ITO)-coated glass surfaces. The main focus of the present study is to control the size of the nanoparticles and study the size-dependent electrocatalytic property of these surface bound Au-Pd nanoparticle nanoparticles to investigate oxidation of higher molecular weight alcohols and polyalcohols that has direct significance for direct alcohol fuel cells (DAFCs).
Research Progress:
A. Two dimensional assemblies of bimetallic Au-Pd nanoparticles:
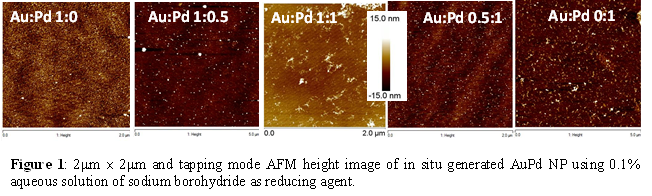
In our present study, we have generated bimetallic AuPd NPs with varying Au:Pd mole ratio of 1:0, 1:0.5, 1:1, 0.5:1, and 0:1. Electrocatalytic activities of these systems have been evaluated for higher molecular weight and polyalcohol oxidation in alkaline medium. Bimetallic AuPd NPs assemblies are generated by simultaneous in situ reduction of [PdCl4]2- with [AuCl4]- ions bound to the silane functionalized surface. AuPd bimetallic NPs generation using 1 ´ 10-2 M HAuCl4 and 1 ´ 10-2 M K2PdCl4 solutions which were made separately and mixed in different volumes to create the desired Au: Pd mole ratio in the final solution. After incubating the surfaces for 8 hours followed by rinsing with water and drying under a flow of argon, the surfaces with adsorbed ions were placed in a freshly prepared 0.1% (w/v) aqueous solution of sodium borohydride for 8 hours to generate the AuPd bimetallic NPs. Representative AFM images shows nearly uniform AuPd NPs on silicon surface (Figure 1) after the final reduction step with the different Au:Pd mole ratio in solution. In this experiment, K2PdCl4 is used as Pd source. An apparent change in the morphology of the nanoparticles is observed with increasing the concentration of palladium ions, the nanoparticles increase in size and number. The average nanoparticle size for Au:Pd 0:1 was ~7.40 nm, for Au:Pd 1:1, the average nanoparticle size was between ~2-3 nm and for Au:Pd 1:0.5 the average nanoparticle size was between ~0.8-1.4 nm as obtained from analysis of a number of AFM images of different samples.
B. Electro-catalytic behavior of bimetallic Au-Pd nanoparticles for polyalcohols:
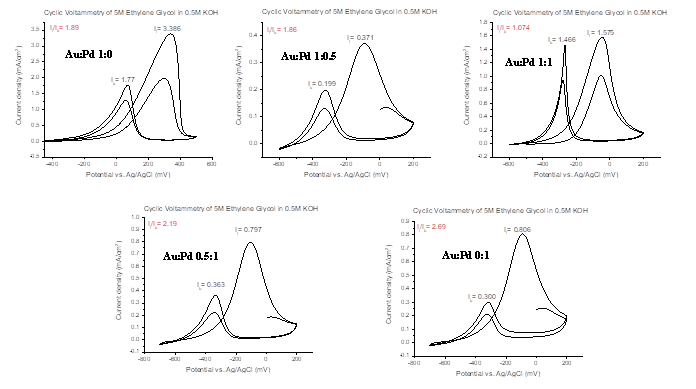
Preliminary results on electrocatalytic activity of AuPdNPs generated by borohydride reduction towards ethylene glycol are presented in Figure 2. A strong symmetric anodic oxidation peak during forward scan is the most noticeable feature in all of the electrocatalytic responses. In addition, all the votammograms show another anodic peak during reverse scan. This anodic peak in the reverse scan is attributed to the removal of the incompletely oxidized carbonaceous species formed in the forward scan. It is also known that accumulation of intermediate carbonaceous material on the catalyst surface leads to catalyst poisoning. Hence the ratio (If/Ib) of the forward anodic peak current density (If) to the reverse anodic peak current density (Ib), can be used to describe the catalyst tolerance of carbonaceous species accumulation. Low If/Ib ratio indicates poor oxidation of specific alcohol during anodic scan and excessive accumulation of carbonaceous residues on catalytic surface. High If/Ib ratio shows greater efficiency of the catalyst. Cyclic voltammetry shows that if the concentration of palladium relative to the concentration of gold increases, then the result is a greater catalytic conversion of ethylene glycol (EG) occurs. In addition we have also used glycerol, methanol and ethanol to assess the catalytic efficiency of the bimetallic catalyst.
Impact of the research
The principal investigator and the undergraduate students are responsible for the execution of the research project. Currently, they are pursuing the project and playing a major role in day-to-day research activities by performing different experiments for advancing the project forward. The undergraduate researchers are involved in mastering the experimental protocols, participating in interpretation and planning of experiments, co-author articles, and presenting their work in scientific meetings.
The present proposed research has provided ample opportunity for undergraduate students to work in the emerging area of nano-structured catalyst. This study involves the use of AFM, and XPS facility in the Electron Microbeam Analysis Laboratory (EMAL) at the University of Michigan-Ann Arbor campus. Apart from the use of microscopy, electrochemical techniques like impedance spectroscopy and cyclic voltammetric measurements with corresponding data analysis is useful for students to understand the fundamentals of electron transfer process at the electrode and also to model the interfacial phenomena associated with it. This is an excellent opportunity for any undergraduate student to get research experience beneficial for future research endeavor. The present funding helped the principal investigator to sustain a contemporary and challenging research program with active participation of undergraduates’ at the Department of Natural Sciences, UM-Dearborn. Moreover, the present funding is continuing to help PI to build a comprehensive nanotechnology/material science program at the Department of Natural Sciences, UM-Dearborn which encompasses the interest and follow the needs of both students and other faculty members.
Student participation, Publications and Presentations:
During this project reporting period 3 undergraduate students have worked on this project. Among these, one is currently applying to dental program and two are in sophomore level. A manuscript is in preparation dealing with AFM, electrochemistry and zeta potential results. One of the students has presented a part of the current work at the recently concluded Fall ACS meeting at Washington D.C.