Reports: DNI1055884-DNI10: Rational Synthesis and Characterization of Non-Layered Two-Dimensional Crystals
Guihua Yu, PhD, University of Texas, Austin
[Scientific Goals]: This ACS-PRF research project aims to develop novel self-assembled two-dimensional (2D) crystals and related nanomaterials, and to better understand the structure-property relationship of these 2D nanostructured solids for applications in energy-related devices.
[Progress]: In the past 2nd year, we have made further significant progress towards the above scientific goal. We recently developed a general synthetic strategy for versatile synthesis of various holey transition metal oxide (TMO) nanosheets with adjustable hole sizes that enable greatly enhanced alkali-ion storage properties. This generic method is based on graphene oxide as sacrificial template for universal synthesis of two-dimensional (2D) holey nanoarchitected TMOs with intrinsically non-layered structures. We also extensively characterized their chemical/crystal structures and physical properties.
We further made important progress towards better understanding of charge transport and storage properties of these novel self-assembled 2D nanomaterials. Detailed electrochemical measurements (e.g. Li+/Na+ storage) of these nanomaterials have been performed and investigated, as well as advanced characterizations such as in-situ transmission electron microscopy (TEM) imaging to understand electrochemical effects have been studied. This leads to better understand of the structure-property relationship of these 2D nanostructured solids for applications in energy storage devices.
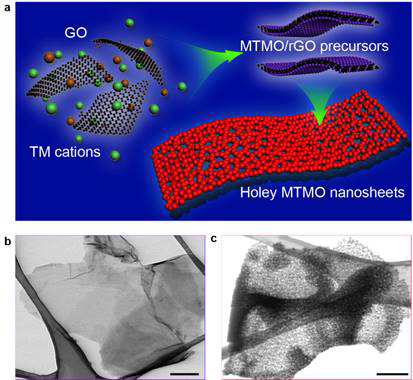
Fig. 1. (a) Schematic showing the general strategy to synthesize 2D holey TMO nanosheets, using graphene oxide (GO) as sacrificial templates. (b) STEM image of ZMO precursor/rGO shows sheets-like morphology. (c) STEM image of 2D holey ZMO nanosheets shows holey nanosheets composed of interconnected ZMO nanocrystals. Scale bar, 200 nm (b, c).
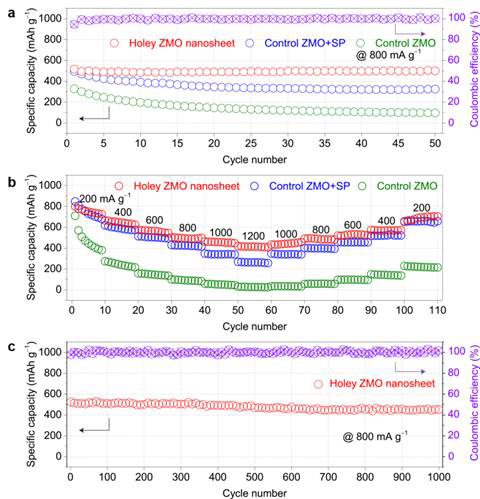
Figure 1 shows the controlled synthesis of 2D holey nanostructured ZnMn2O4 (ZMO) nanosheets with adjustable hole structures via this GO-sacrificial-template method. By tuning the synthetic concentration of precursor chemicals and post-calcination temperature, different hole size/density could be readily achieved. Fig. 2 compares Li-ion storage properties of three different samples (holey nanosheets, nanoplates without holes, and particles) to show different electrochemical transport and storage kinetics. 2D holey ZMO nanosheets showed superior rate capability and cycling stability (Fig. 2). This comprehensive study may provide an interesting material model for detailed investigation of the fundamental relationship between the structures and properties.
Fig. 2. Lithium-ion storage properties of 2D holey ZMO nanosheets. (a) Cycling tests of 2D holey ZMO nanosheets, control ZMO+SP, and control ZMO at current density of 800 mA/g . (b) Rate capability of 2D holey ZMO nanosheets, control ZMO+SP, and control ZMO at different current densities. (c) Long-term cycling stability and Coulombic efficiency of 2D holey ZMO nanosheet at current density of 800 mA/g.
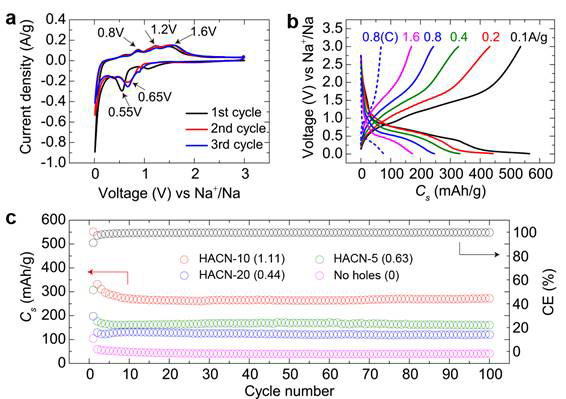
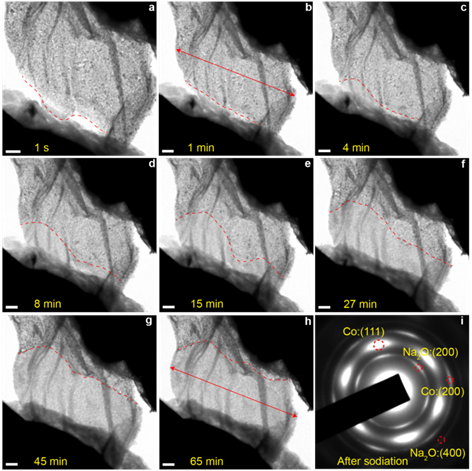
Another example is on controlled holey Co3O4 nanosheets and the detailed study on their sodium-ion storage properties (Fig. 3). The interconnected 2D nanostructured Co3O4 shows greatly improved alkali-ion storage characteristics. In-situ TEM characterization (Fig. 4) was employed to depict the structural evolution and further understand the structural stability of these 2D holey architectures during the sodiation process.
Fig. 3. Electrochemical characterization of holey Co3O4 nanosheets for sodium ion storage: (a) CV curves; (b) Charge/discharge curves at various current densities,; (c) Cycling stability of holey Co3O4 nanosheets with different pores at 0.8 A/g for 100 cycles.
Fig. 4. In-situ sodiation process of 2D holey Co3O4 nanosheets: (a-h) typical morphological evolution, and the red dash line shows the sodiation front (conversion phase boundary) and the red arrows show the volume expansion. (i) Diffraction pattern of the phase after full sodiation. Scale bar in a-h is 200 nm.
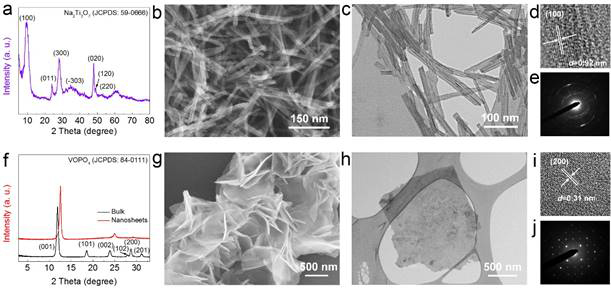
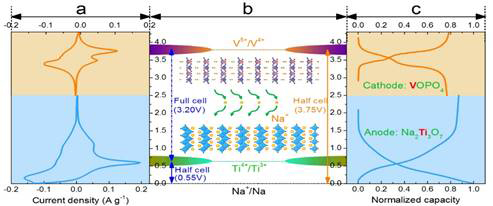
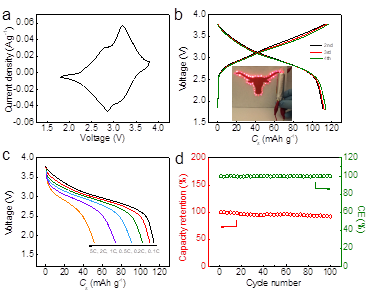
In addition to developing novel synthetic approaches to synthesize 2D nanostructured crystals and their alkali-ion storage properties, we also extended our research efforts to exploring the challenges and material design for sodium-ion full batteries. Recently we rationally designed a full sodium-ion battery (SIB) based on nanostructured Na2Ti3O7 and VOPO4 (layered materials) as the anodes and cathodes (Fig. 5), owing to their advantageous electrochemical features (Fig. 6). We demonstrated a SIB with one of the highest operating voltages ~3 V and outstanding rate capability and excellent cycling stability. An exceptionally high energy density of 220 Wh/kg is achieved, which is comparable to the state-of-the-art LIBs. (Fig. 7). Layered crystalline structured Na2Ti3O7 nanotubes and VOPO4 nanosheets were systematically investigated and compared with other electrodes reported in existing literature for building next-generation sodium ion full cells. This marks a step forward in the field as the asymmetric sodium ion full cell we designed shows excellent reversible capacity, rate capability and cycle stability.
Fig. 5. Morphological and structural characterization of Na2Ti3O7 and VOPO4 samples. XRD patterns of Na2Ti3O7 nanotubes (a) and exfoliated VOPO4 nanosheets (f). SEM images for the Na2Ti3O7 nanotubes (b) and VOPO4 nanosheets (g). HRTEM images and associated SAED patterns of the Na2Ti3O7 (c, d, e) and VOPO4 (h, I, j).
Fig. 6. Electrochemical characteristics of Na2Ti3O7 and VOPO4 electrodes in the half cell. (a) CV curve of VOPO4 (orange) and Na2Ti3O7 + (blue) vs. Na+/Na. (b) Schematic of potential of the V5+/V4+ redox couple in layered Na2Ti3O7 and Ti4+/Ti3+ redox couples in layered VOPO4 materials. (c) Typical charge-discharge profiles of the VOPO4 for V5+/V4+ (orange) and Na2Ti3O7 for the Ti4+/Ti3+ (blue) vs. Na+/Na.
Fig. 7. Electrochemical performance of Na2Ti3O7//VOPO4 full cells. a) CV curves profiles. b) Typical charge/discharge profiles at a rate of 0.1C. c) The capacity at different rates of 0.1C, 0.2C, 0.5C, 1C, 2C and 5C. d) cycling stability.
[Significance and Impact]: To summarize, we have made significant progress towards both controlled synthesis of novel 2D nanostructured crystals (especially those with intrinsically non-layered structures), and better understanding of electrochemical properties of these self-assembled 2D nanostructured materials for energy storage applications. Our research progress laid foundation for the future work on developing bottom-up controlled synthesis of 2D crystals for various types of materials to enable ‘new materials by design’ for emerging energy technologies. The graduate students working on this project have also gained extensive training in synthesis and characterization of nanostructured solids, and are making great research progress. Under ACS-PRF support, one graduate student has recently graduated with a PhD degree in Material Science and Engineering, and the other is on the right track towards PhD.