Reports: DNI656892-DNI6: Molecular Mechanisms of Corrosion Inhibitors Studied Using Molecular Simulations
Sumit Sharma, PhD, Ohio University
Corrosion inhibitors are surfactant molecules which are injected into oil and gas pipelines in small quantities to inhibit internal corrosion due to the presence of water and ionic species. These molecules are known to mitigate corrosion by forming ordered adsorbed layers on metal surfaces. The objective of this research is to understand factors which govern adsorption and self-assembly of corrosion inhibitor molecules on metal surfaces. We are studying this problem via three approaches as discussed below:
(a) Study how molecular properties affect adsorption and self-assembly of corrosion inhibitor molecules
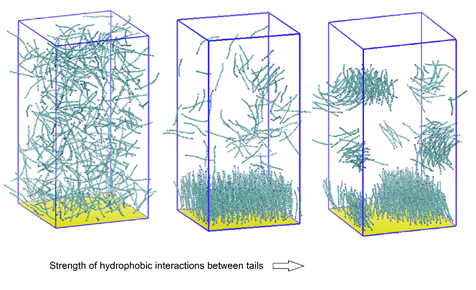
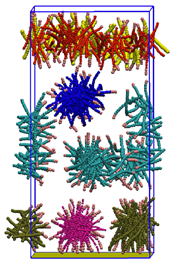
In this study, we employ a coarse-grained model. A corrosion inhibitor molecule is represented by a linear, bead-spring model with a polar “head” bead and a chain of hydrophobic “tail” beads. A smooth surface, strongly attractive to the polar beads, represents the metal surface. This model is based on the understanding that the polar group of corrosion inhibitor molecules has strong affinity for metal surfaces. We systematically vary the strength of hydrophobic interactions between the hydrophobic tails and the size of the polar head group and investigate how these properties affect adsorption characteristics. Our main findings are that (1) hydrophobic interactions between adsorbed molecules promote adsorption and self-assembly; and (2) the morphology of the adsorbed layer is governed by the geometry of the molecules. Fig. 1 shows how the equilibrium adsorbed state changes from low, random adsorption for the case of weak hydrophobic interactions to formation of a self-assembled monolayer (SAM) at moderate hydrophobicity values. When the hydrophobic interactions are strong, the molecules form aggregates in the bulk and the adsorbed phase, which leads to a decrease in the adsorbed amount. When the polar head bead is taken twice the size of the hydrophobic beads, the molecules form cylindrical micelles in the bulk and the adsorbed phase instead of a SAM (Fig. 2). For all cases, we find that the adsorbed phase has significant fraction of molecules with their polar head group away from the surface, indicating that the adsorbed layer of corrosion inhibitor molecules is patchy. These results corroborate with experimental observations and provide many new insights into the adsorption behavior and the molecular nature of the adsorbed layers.
(b) Study of transport and aggregation characteristics of corrosion inhibitor molecules in bulk aqueous solutions and their adsorption on to metal surfaces via atomistic molecular dynamics (MD) simulations
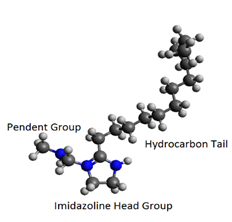
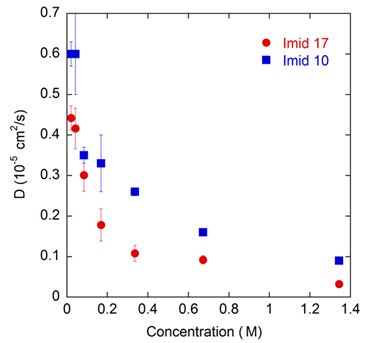
The goal of this study is to understand diffusion and aggregation properties of imidazoline-based corrosion inhibitors in aqueous solutions as function of their chemistry, as well as study their adsorption on to metal-water interfaces. We study a protonated imidazoline-based surfactant with a 10 carbon long (imid 10) and a 17 carbon long (imid 17) alkyl chain (Fig. 3) in explicit water. In MD simulations, we observe the corrosion inhibitor molecules to aggregate in the bulk, which corresponds to a significantly reduction in their diffusion (Fig. 4). The molecules aggregate as micelles with a hydrophobic core and polar group exposed to the solution. Corrosion inhibitor molecules in infinite dilution adsorb on to metal surfaces with their alkyl chains parallel to the surface. At higher concentrations, wherein the molecules form aggregates, no significant adsorption on the metal surfaces is observed. We are performing potential of mean force (PMF) calculations of adsorption of an imid17 and imid10 molecule on metal surfaces. Furthermore, we are also examining the role of surface charge in the adsorption behavior of these molecules on the surfaces.
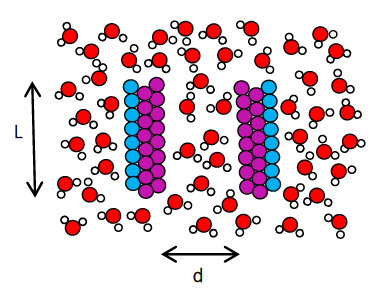
(c) Behavior of water under hydrophobic confinement
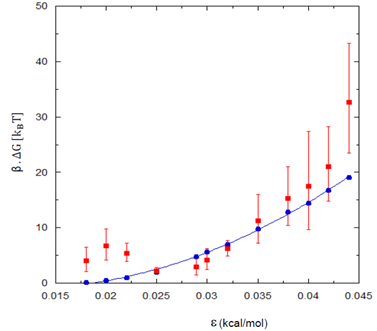
Hydrophobic effect, the phenomenon of aggregation of non-polar species in water, is governed by the behavior of water in the vicinity of non-polar solutes. In this work, we investigate how the stability of water confined between two hydrophobic surfaces depends on the chemistry of the surfaces. The hydrophobic surfaces are represented as three layers of Lennard-Jones (LJ) atoms arranged in a hexagonal lattice (Fig. 5). The two hydrophobic surfaces are kept fixed, parallel to each other at a certain distance, d from each other. The hydrophobic character of the surface is altered by changing the LJ energy parameter, ε. Fig. 6 shows the calculated free energy barriers to evaporation of water confined between the surfaces with d = 1.4 nm and lateral size of the surfaces, L = 3 nm, as a function of ε. The free energy barriers are calculated using Umbrella sampling. The magnitude of free energy barriers are also estimated using macroscopic nucleation theory and decent agreement is observed.
The funding from ACS PRF has been a tremendous positive impact on our research. As is demonstrated through the projects discussed above, our research has grown in multiple directions. We have presented our research work in numerous conferences (American Institute of Chemical Engineers annual conference, American Physical Society, Midwest Statistical Mechanics Conference). We have submitted one manuscript for publication on this work and are in the process of writing two more manuscripts. One student, whose MS thesis work was sponsored by this grant, has successfully defended his MS thesis and is pursuing PhD at the University of Michigan Ann Harbor. We have also started investigating electronic interactions between polar groups of corrosion inhibitor molecules and metal surfaces using density functional theory.
Fig. 1. Adsorption behavior as a function of strength of hydrophobic interactions between tails of corrosion inhibitor molecules.
Fig. 2. Cylindrical micelles of corrosion inhibitor molecules observed in both adsorbed and bulk phases. The inhibitor molecules have polar head group of size twice that of hydrophobic monomers.
Fig. 3. An imidazoline-based corrosion inhibitor molecule
Fig. 4. Diffusion coefficient of imidazoline based corrosion inhibitors as a function of concentration
Fig. 5. Schematic showing simulation system to study the behavior of water in hydrophobic confinement.
Fig. 6. Free energy barrier to evaporation, βΔG as a function of LJ interaction parameter ε between surface atoms and water oxygen atoms. The blue solid line represents free energy barriers predicted from macroscopic nucleation theory.