Reports: ND354954-ND3: N-Heterocyclic Olefins (NHOs) as Tunable Ligands for Metal-Mediated Catalysis
Eric Rivard, PhD, University of Alberta
During this PRF supported project, efficient routes to an emerging class of carbon-based donor, termed by us as N-heterocyclic olefins (NHOs), have been developed. With this library of >20 new NHO ligands, we explored palladium-catalyzed cross-coupling protocols for C-C and C-N bond formation. Our hindered NHO ligands are also very active hydroborylation organocatalysts.
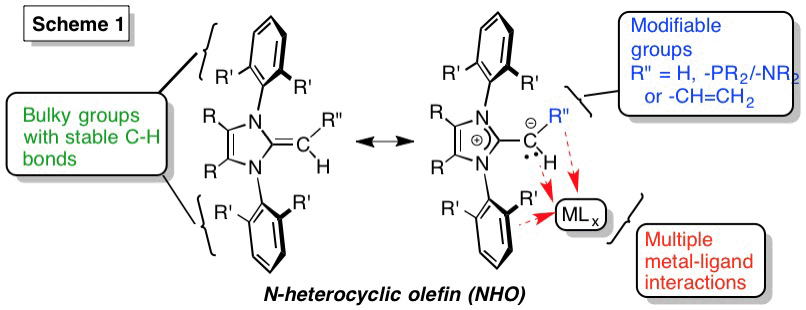
NHOs have been known since the early 1990s[1] and due to the presence of a highly polarized exocyclic double bond, these species can act as Lewis bases (Scheme 1). After our group's use of NHOs to stabilize low-valent main group compounds (e.g. GeH2) in 2011,[2] more than a dozen groups now investigate NHOs, including in organocatalysis and polymer synthesis.[3]
Phase 1a: Monodentate NHOs
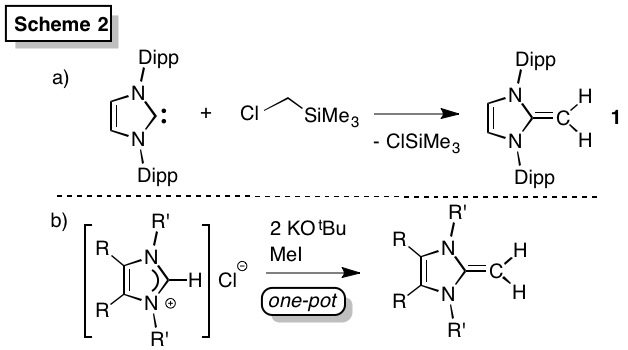
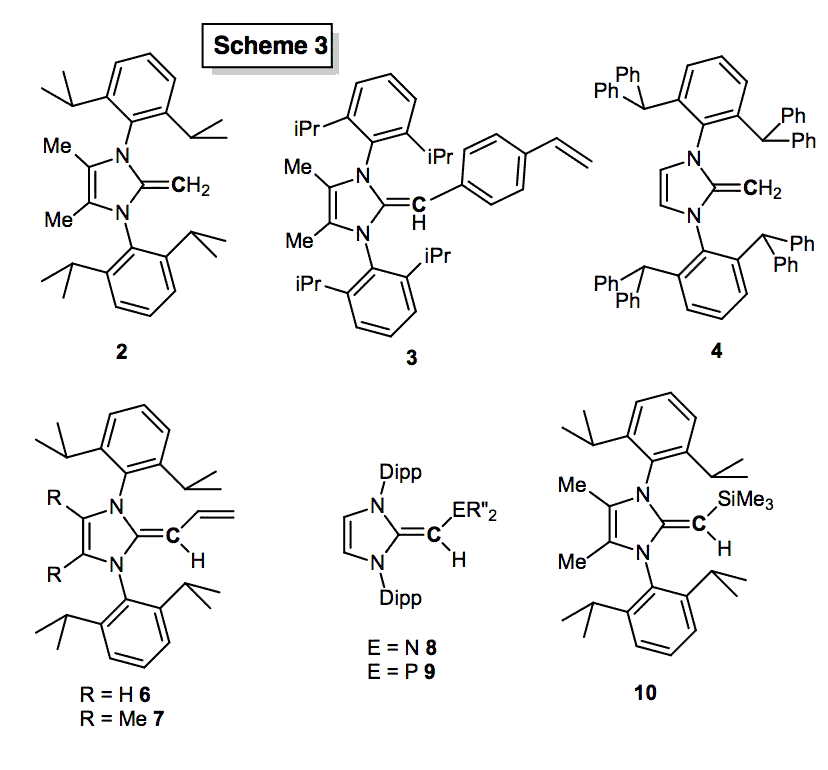
To facilitate the widespread use of NHOs, improved syntheses were first established. A general one-pot synthesis of a wide range of NHOs was achieved (Scheme 2b), including those with polymerizable side groups (3, Scheme 3). The most studied NHO IPrCH2 [1, IPr = (HCNDipp)2C:, Dipp = 2,6-iPr2C6H3] is now obtained on a > 20 g scale by combining the free carbene IPr with ClCH2SiMe3 (Scheme 2; PRF publication; Powers et al.). As hindered donors often perform best in cross-coupling, the exceedingly bulky NHO, IPr*CH2 (4) (Scheme 3) was also prepared.
Bicyclic NHOs with alkane rings fused at the terminal carbon donor sites were synthesized (5, Scheme 4). These donors have steric bulk directly at the site of ligation. As in the Buchwald diarylphosphines, there exists the possibility for stabilizing secondary aryl group---metal interactions within 5 (Scheme 4).
Phase 1b: Bidentate NHOs
Bidentate NHOs were also prepared, including the butadiene analogues 6 and 7 (Scheme 3). These ligands are conceptually related to the widely used allyl ligands, such as cinnamyl (Cin), found in highly active Pd pre-catalysts. We also prepared NHO units fused to both hard amine (-NMe2) and soft phosphine (-PPh2 and –PiPr2) donors (8 and 9, Scheme 3; Dalton Trans.; Paisley et al.). The idea is to access to hard/soft donor combinations to stabilize various metal oxidation states found in catalysis.
Phase 1c: Main Group and Organocatalysis via NHOs
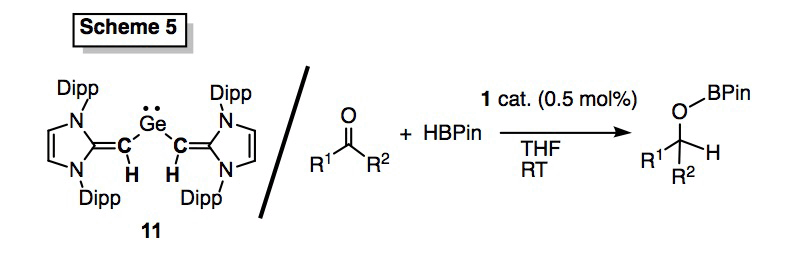
We also accomplished the deprotonation of a =CH2 terminated NHO, e.g. IPrCH2, to give [IPr=CH]- ligands with dual sigma and pi-donating abilities. As shown in Scheme 5 two anionic NHO units were installed onto Ge to give a rare two-coordinate germylene (11) which is being explored as a metal-free hydrogenation catalysts (Angew. Chem., Int. Ed.; Hering-Junghans et al.). We also found that NHO 1 is an exceedingly active catalyst for the hydroborylation of hindered ketones (Scheme 5; Dalton Trans.; Hering-Junghans et al.)
Phase 2: Donor Properties of NHOs
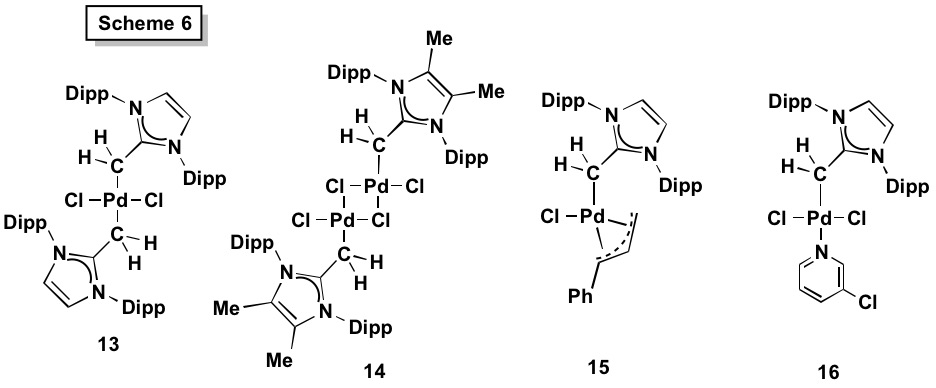
We also prepared various NHO Pd(II) complexes with representative examples shown in Scheme 6. Dimeric complexes [(NHO)PdCl2]2 (e.g. 14) were obtained directly from [PdCl2(NCPh)2] and NHO, while known procedures were also used to prepare the active catalyst [(IPrCH2)PdCl(Cin)] (15) and PEPPSI analogues such as [IPrCH2)PdCl2(3-chloropyridine)] (16); such complexes with NHCs as ligands have been shown to be highly effective pre-catalysts in Buchwald-Hartwig and Suzuki-Miyaura cross coupling protocols.[4] It was found that Pd(II) centers in the presence of base (KOtBu and OAc-) led to C-H deprotonation of the backbone of the NHO 1; thus out catalytic investigations were focused on Me-backbone functionalized NHOs, such as 2 (Scheme 3).
Tolman Electronic Parameters (TEPs) of our NHOs were determined via IR analysis of the Rh(I) complexes [(NHO)RhCl(CO)2] (17). The lower v(CO) values in 17 compared to the carbene (NHC) analogues suggested that NHOs are better donors than NHCs, however our ligand displacement and computational studies show a more subtle reason. NHCs are good s-donors but also reasonable pi-acceptors (leading to higher ν(CO) than expected); however NHOs are moderate sigma-donors but quite poor pi-acceptors due to a lack of low-lying LUMO orbitals.
Phase 3: Catalyst Screening/Cross-Coupling
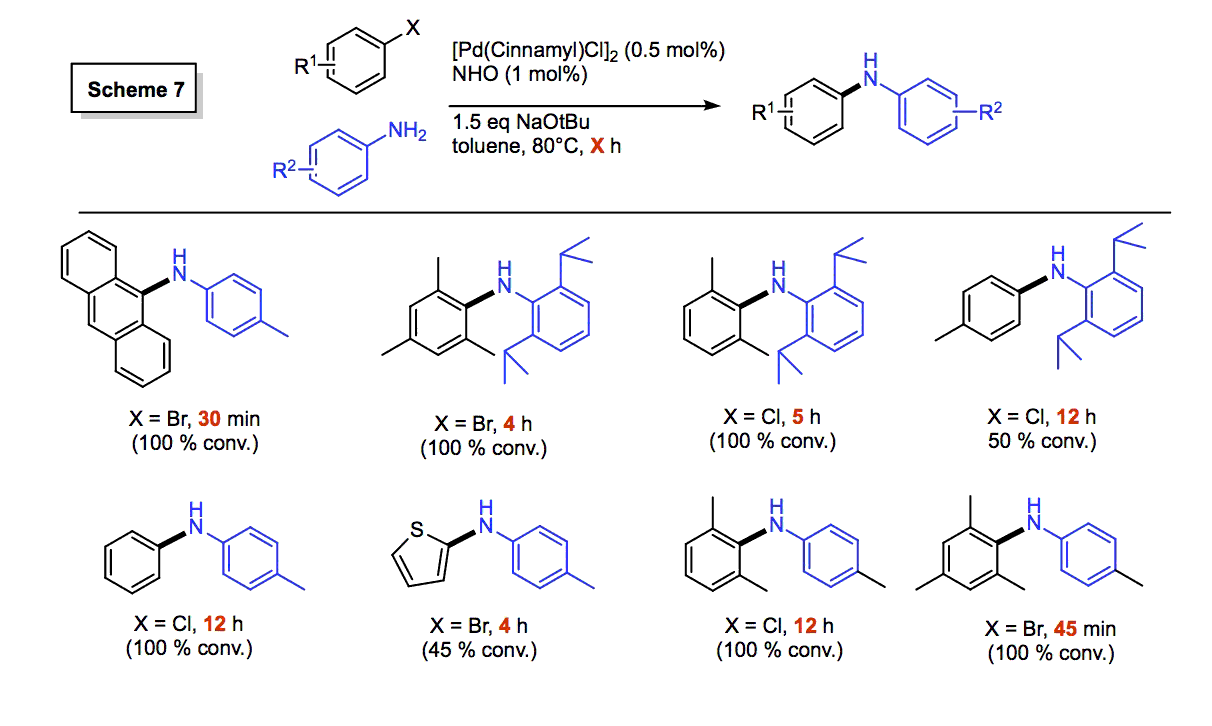
We tested various mixtures of NHOs (Scheme 3) with Pd(0) and Pd(II) sources in 0.5 to 5 mol% amounts in 1,4-dioxane, THF, toluene or DME with NaOtBu as an external base in C-N bond forming reactions; each reaction was monitored by 1H NMR spectroscopy using 1,3,5-(Me3C6H3) as an internal standard. Unlike the case of N-heterocyclic carbenes (NHCs), our NHOs did not form catalytically competent mixtures with the Pd(0) precursors (e.g. Pd(PPh3)4), presumably due to a lack of pi-accepting ability in NHOs which led to the formation of copious amounts of bulk metal. To our delight, in situ generation of the [(NHO)PdCl(Cin)] pre-catalysts (Cin = cinnamyl) enabled Pd-loadings as low as 0.5 mol%, and our new NHO catalysts could be used to couple arylchlorides and very hindered arylamines (Scheme 7), generally considered as unreactive substrates; the catalytic activity rivaled that found with known NHC ligands.[4] We also noted that overarylation (to yield Ar3N products) does not happen at all with our NHO ligands, thus providing enhanced control versus NHC systems.
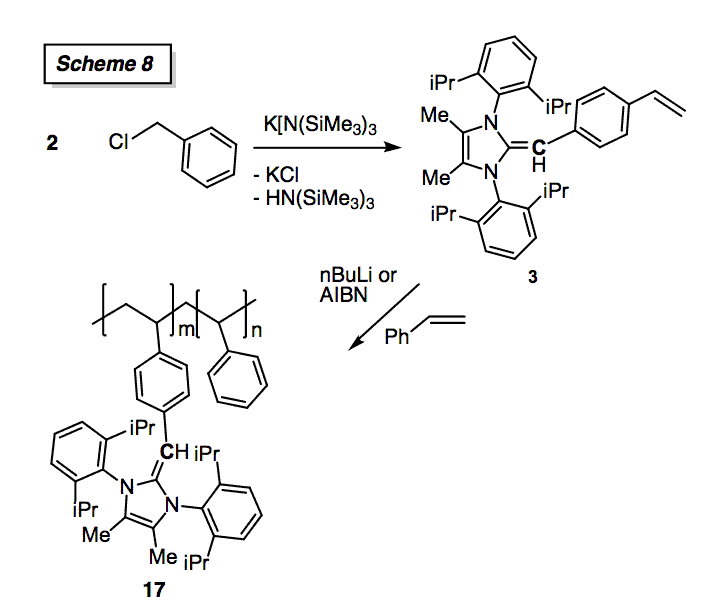
Given our prior experiences with using Suzuki-Miyaura C-C cross-coupling to prepare low-band gap polymers[5] we decided to study the abovementioned Pd complexes for this transformation. We explored the coupling of arylboronic acids with various arylbromides, and noted disappointed performances (only partial conversions) with most Pd-NHO pre-catalysts; in general activities of PEPPSI-type systems (NHO)PdCl2(3-chloropyridine) (16) were about half of those found in Organ's widely used NHCs complexes. After much effort we were able to find a general route to append polymerizable styrene groups onto an NHO (3, Scheme 8); related chemistry is quite challenging for N-heterocyclic carbene systems, thus showing one advantage of our NHOs. Moreover we showed that 3 can bind Pd via the internal olefin, thus opening the door for supported catalysis using the new copolymer 17 (Scheme 8). We have also found that the hybrid NHO-phosphine 9 (R = Ph) is a competent ligand for C-N bond formation, and we are exploring more hindered analogues in this regard.
References
[1] Kuhn et al. Chem. Ber. 1994, 127, 1405; [2] Rivard et al. Chem. Commun. 2011, 47, 6987; [3] Dove et al. ACIE 2015, 54, 9550; [4] Organ et al. ACIE 2009, 48, 2383; [5] Rivard et al. JACS, 2013, 135, 5360.