Reports: UNI354833-UNI3: Multifunctional Bipyridine Ligands for the Catalytic Reduction of CO2 to CO
Sheri Lense, PhD, University of Wisconsin Oshkosh
Carbon monoxide (CO) can be used as a starting material for many valuable chemicals. One promising way to produce CO is via the reduction of carbon dioxide (CO2) produced from petroleum usage. However, reduction of CO2 to CO is a formidable challenge, and a suitable catalyst is required for this reaction to occur with enough speed, efficiency and selectivity. When [M(CO)3(R-bpy)X]-type complexes (M = Re or Mn, R-bpy = bipyridine or bipyridine derivative, and X = halide) are reduced in situ they can catalyze reduction CO2 to CO with good selectivity.1 For most CO2 reduction catalysts of this type, catalysis occurs following two one-electron reductions of the [M(CO)3(R-bpy)X] complex. The reduction pathway involves protonation of CO2, and recently addition of an intramolecular Brønsted acid in the form of a phenol substituent on the bipyridine ligand of a [Mn(CO)3(R-bpy)Br] catalyst was found to result in an enhanced catalytic current at a moderate overpotential.2 Support from the ACS PRF is enabling us to further investigate the effect of the pKa of this intramolecular Brønsted acid substituent and the effect of varying the number of intramolecular Brønsted acid substituents on the rate, selectivity, and efficiency of CO2 reduction catalysis.
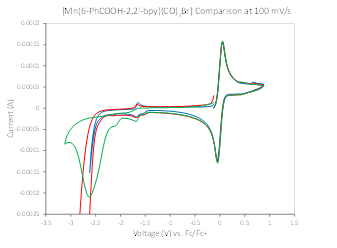
In this project, we are investigating how to optimize the intramolecular Brønsted acid for optimal catalytic reduction of CO2 to CO. One line of inquiry involves investigation of the effect of the intramolecular acid strength on catalytic rate, efficiency, and selectivity. To address this question, we first synthesized a ligand in which the phenol substituent studied previously2 was replaced by a benzoic acid substituent. The pKa of phenol and benzoic acid in acetonitrile have been determined to be 29.14 and 21.51,3 respectively. This ligand, termed 6-PhCOOH-2,2’-bpy (Figure 1), was metallated to form [Mn(6-PhCOOH-2,2’-bpy)(CO)3Br] and [Re(6-PhCOOH-2,2’-bpy)(CO)3Cl]. The resulting complexes were characterized spectroscopically and by single molecule X-ray diffraction. To our knowledge, these are the only metal complexes utilizing the 6-PhCOOH-2,2’-bpy or similar ligand in which the benzoic acid group is protonated and not coordinated to the metal. While the cyclic voltammetry (CV) of this complex under an inert gas is similar to that of other [M(CO)3(R-bpy)X]-type complexes, in the presence of CO2 and water no catalytic wave is observed following the two electron reduction of either the Mn or Re complex (Figure 2). To probe whether inhibition of catalysis was specifically caused by the intramolecular benzoic acid substituent, the CV of [Mn(bipyridine)(CO)3Br] was measured with up to 50-fold excess intermolecular benzoic acid. No reduction of catalysis was observed in the presence of the added benzoic acid, indicating that it is the intramolecular nature of the benzoic acid substituent that is inhibiting catalysis.
Figure 1. 6-PhCOOH-2,2’-bpy
Figure 2. CVs performed in acetonitrile (MeCN) solvent, 0.2M tetrabutylammonium PF6 (TBAPF6) electrolyte, and referenced to ferrocene/ferrocenium, under a N2 atmosphere (blue trace), under N2 with 5% water (red trace), and under CO2 with 5% water (green trace).
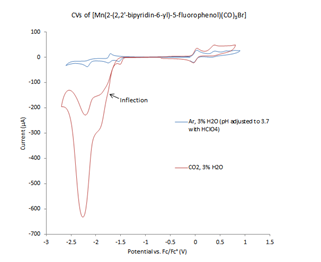
We pursued the investigation of the effect of intramolecular acid pKa on catalysis by adding an electron-withdrawing group to the phenol substituent. Attempts to synthesize a trifluoromethyl-substituted ligand resulted in cleavage of this group during the final deprotection step in the ligand synthesis. However, we were able to synthesize a bipyridyl ligand containing a 3-fluorophenol substituent, 2-(2,2’-bipyridin-6-yl)-5-fluorophenol (Figure 3). In water, the pKa of 3-fluorophenol is 9.29 whereas that of phenol is 9.99.4 The ligand was metallated to form [Mn(2-(2,2’-bipyridin-6-yl)-5-fluorophenol)(CO)3Br], which was characterized spectroscopically and by elemental analysis. The CV of this complex was performed under both an inert atmosphere and in the presence of CO2 and water to investigate its catalytic properties. Under catalytic conditions, the CV of [Mn(2-(2,2’-bipyridin-6-yl)-5-fluorophenol)(CO)3Br] is complex (Figure 4). Like several other [M(CO)3(R-bpy)X]-type complexes, a catalytic wave is observed at the second one-electron reduction. However, this catalytic wave has an inflection, indicating a change in catalytic mechanism or another reduction event as the potential becomes more negative. Additionally, a second catalytic wave appears at more negative potentials that has a much greater magnitude in the presence of CO2 and water than in the presence of argon and pH-adjusted water. Current and future work involves the characterization of the catalytic products following controlled potential electrolysis and further investigation of the electrocatalytic mechanisms. Additionally, we are synthesizing a ligand containing a phenol with an electron-donating substituent for comparison.
Figure 3. 2-(2,2’-bipyridin-6-yl)-5-fluorophenol
Figure 4. CVs performed in acetonitrile (MeCN) solvent, 0.2M tetrabutylammonium PF6 (TBAPF6) electrolyte, and referenced to ferrocene/ferrocenium, under an argon atmosphere with 3% H2O (pH adjusted to 3.7 with HClO4) (blue trace) and CO2 with 3% H2O (red trace).
Our second line of inquiry involves the synthesis of bipyridyl ligands containing resorcinol or multiple phenol substituents, in order to investigate how the number of intramolecular Brønsted acid substituents affects catalysis. These complexes have been successfully synthesizing and are currently undergoing electrochemical analysis.
This research involved numerous undergraduate students at the University of Wisconsin Oshkosh, all of whom experienced fundamental scientific research for the first time through working on this project. Working on the research project allowed these students to learn synthetic, spectroscopic, and analytical techniques beyond what is covered in the undergraduate curriculum. In addition to performing experiments students became involved in trouble-shooting problems encountered during synthesis and analysis, data interpretation, and experimental design. Students also presented posters of their work at university-wide research events. This grant has greatly benefitted my career, allowing me to establish a research program in a cutting-edge field and perform research with several talented undergraduates to investigate important questions regarding design of catalysts that convert CO2 ultimately to value-added chemicals.
References
1. (a) Takeda, H. et al. JACS 2008, 130 (6), 2023–2031; (b) Bourrez, M. et al. Angewandte Chemie Int. Ed. 2011, 50 (42), 9903-9906.
2. Agarwal, J. et al. Inorg. Chem. 2015, 54, 5285-5294.
3. Muckerman, J.T. et al. Biochim. Biophys. Acta 2013, 1827(8-9), 882-91.
4. CRC Handbook of Chemistry and Physics, 84th ed.; Lide, D.R., Ed.; CRC Press: Boca Raton, FL, 2003; Section 8, p 51.