Reports: UR152798-UR1: Synthesis of Azaannulenes Related to the Porphyrins
Timothy D. Lash, PhD, Illinois State University
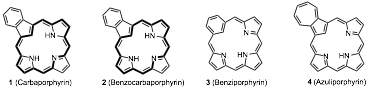
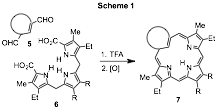
Porphyrins are macrocyclic systems built up from four pyrrolic subunits that possess global aromatic properties. We have investigated the synthesis of closely related porphyrin analogues where one or more of the usual pyrrolic subunits have been replaced by carbocyclic rings. Although these carbaporphyrinoid systems may retain aromatic characteristics, in some cases these analogues are nonaromatic or even antiaromatic.1,2 True carbaporphyrins such as 1 and 2 exhibit the strong diamagnetic ring currents associated with aromatic systems,1,3 while benziporphyrins 3 are essentially nonaromatic4 and azuliporphyrins 4 have intermediary properties.5 These systems exhibit unusual reactivity, undergoing selective oxidation reactions and generating stable organometallic derivatives under mild conditions.6 In addition, derivatives of benzocarbaporphyrins 2 have been shown to be effective agents in the treatment of leishmaniasis.7 A "3 + 1" variant on the MacDonald reaction, where an aromatic dialdehyde 5 is condensed with a tripyrrane 6 in the presence of TFA, has commonly been used to prepare carbaporphyrinoids (Scheme 1).8 This strategy has been very successful, and has provided straightforward access to carbaporphyrinoids such as 1-4.1,2 This methodology has recently been adapted to prepare new classes of carbaporphyrinoid systems.9,10
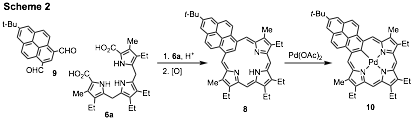
The "3 + 1" strategy was applied to the synthesis of a polycyclic aromatic hydrocarbon (PAH)-porphyrin hybrid. Syntheses of structures of this type have recently attracted a considerable amount of attention.11 Pyreniporphyrin 8 was prepared by condensing pyrene dialdehyde 9 with tripyrrane 6a in the presence of trifluoroacetic acid (Scheme 2).9 The PAH-porphyrin hybrid proved to be nonaromatic in the free base form, but upon protonation the related dication showed significant diatropic character as assessed by proton NMR spectroscopy, NICS calculations and ACID plots.9 Reaction with palladium(II) acetate in refluxing acetonitrile gave a stable palladium(II) derivative 10. A related thiapyreniporphyrin was also investigated.
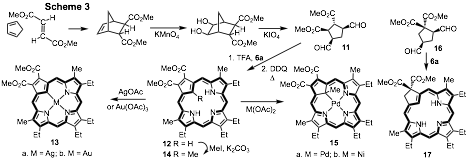
Most studies on true carbaporphyrins have been conducted on benzo-fused structures such as 2. Very recently, we reported a greatly improved route to carbaporphyrins and carbachlorins that do not possess fused aromatic rings and these new systems show great promise for future investigations.10 A cyclopentane dialdehyde 11 was prepared in two steps from the Diels-Alder adduct of cyclopentadiene and dimethyl fumarate (Scheme 13). Condensation with tripyrrane 6a in the presence of TFA, followed by oxidation with DDQ in refluxing toluene, gave the carbaporphyrin diester 12 in 40% yield together with <10% yield of a related carbachlorin.10 The electron-withdrawing ester moieties appear to stabilize the porphyrinoid, and this property has enabled the synthesis of silver(III) and gold(III) derivatives 13a and 13b. Alkylation with MeI and K2CO3 afforded 21-methylcarbaporphyrin 14 and this reacted with palladium(II) or nickel(II) acetate to give good yields of the stable organometallic derivatives 15a and 15b. A cyclopentane dialdehyde 16 with geminal diester units was similarly prepared and this reacted with tripyrrane 6a to give carbachlorin 17. Further oxidation is blocked by the presence of the geminal substituents. Carbachlorins are of interest because they have moderately strong absorptions near 650 nm. Reaction with silver(I) acetate afforded the related silver(III) complex.10
References
1. Lash, T. D. Recent advances in the synthesis and chemistry of carbaporphyrins and related porphyrinoid systems, Eur. J. Org. Chem. 2007, 5461-5481.
2. Lash, T. D. Carbaporphyrinoid Systems. Chem. Rev. 2017, 117, 2313-2446.
3. Li, D.; Lash, T. D. Synthesis and reactivity of carbachlorins and carbaporphyrins. J. Org. Chem. 2014, 79, 7112-7121.
4. Lash, T. D. Benziporphyrins, a unique platform for exploring the aromatic characteristics of porphyrinoid systems, Org. Biomol. Chem. 2015, 13, 7846-7878.
5. Lash, T. D. Out of the blue! Azuliporphyrins and related carbaporphyrinoid systems Acc. Chem. Res. 2016, 49, 471-482.
6. Lash, T. D. Metal complexes of carbaporphyrinoid systems. Chem. Asian J. 2014, 9, 682-705.
7. Taylor, V. M.; Cede–o, D. L.; Mu–oz, D. L.; Jones, M. A.; Lash, T. D.; Young, A. M.; Constantino, M. H.; Esposito, N.; VŽlez, I. D.; Robledo, S. M. In Vivo and In Vitro Studies of the Utility of Dimethyl and Diethyl Carbaporphyrin ketals in the Treatment of Cutaneous Leishmaniasis. Antimicrob. Agents Chemother. 2011, 55, 4755-4764.
8. Lash, T. D. What's in a Name? The MacDonald Condensation. J. Porphyrins Phthalocyanines 2016, 20, 855-888.
9. Gao, R.; AbuSalim, D. I.; Lash, T. D. Pyreniporphyrins, porphyrin analogues that incorporate a polycyclic aromatic hydrocarbon subunit within the macrocyclic framework. J. Org. Chem. 2017, 82, 6680-6688.
10. Sahota, N.; Ferrence, G. M.; Lash, T. D. Synthesis and properties of carbaporphyrin and carbachlorin dimethyl esters derived from cyclopentanedialdehydes. J. Org. Chem. 2017, 82, 9715-9730.
11. Stepien, M.; Gonka, E.; Zyla, M.; Sprutta, N. Heterocyclic nanographenes and other polycyclic heteroaromatic compounds: synthetic routes, properties, and applications. Chem. Rev. 2017, 117, 3479-3716.