Reports: DNI156158-DNI1: New Organocatalysts for Chemoselective Aliphatic C-H Hydroxylation
Michael K. Hilinski, PhD, University of Virginia
Our objective during this funding period was the development of dioxirane-mediated, ketone-catalyzed methods for the hydroxylation of aliphatic C-H bonds that proceed with scope and selectivity similar to that achieved when dioxiranes such as methyl(trifluoromethyl)dioxirane (TFDO) are used as isolated reagents. The expected advantages of the catalytic approach are an elimination of the need for performing a rather laborious preparation of the reagent TFDO, and the introduction of ketone catalyst design as a strategy to address issues of selectivity in C-H hydroxylation.
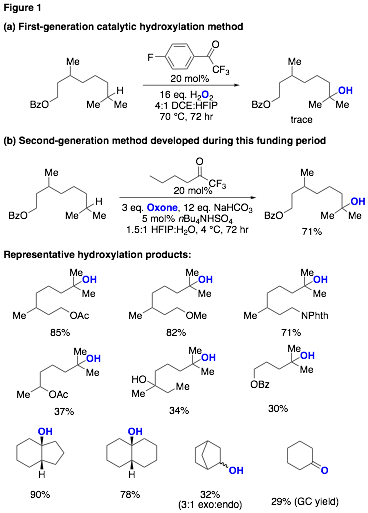
During this first year of the award significant progress has been achieved towards the above goal. Previously, we had developed a method for ketone-catalyzed C-H hydroxylation that employed trifluoroacetophenones as catalysts and aqueous hydrogen peroxide as the terminal oxidant (Figure 1A). While this method is notable for being the first example of dioxirane-mediated, ketone-catalyzed hydroxylation, the substrate scope did not match that observed using isolated TFDO. An important insight acquired during this earlier study has led to the more recent development of a second-generation catalytic method that more closely approximates results achieved using pre-prepared dioxiranes. Specifically, it was observed for the previous method that the use of hexafluoro-2-propanol (HFIP) as a cosolvent was required to achieve the lowest catalyst loadings and highest yields. In the development of the second-generation catalytic method, we reexamined the use of more typical conditions for dioxirane formation (e.g. Oxone/NaHCO3) using HFIP as a cosolvent. These modified conditions, combined with the use of a less sterically encumbered trifluoromethyl ketone, allowed for the development of a catalytic method that exhibits substrate scope and selectivity that is greatly improved from the previous method and that is consistent with that achieved using isolated TFDO (Figure 1B).
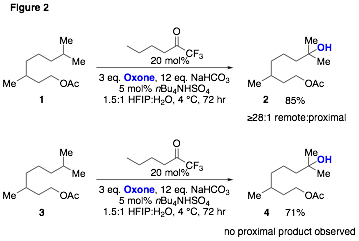
The application of this new chemistry to a variety of substrates revealed advantages of this catalytic method beyond eliminating the need to use isolated TFDO. For example, although the use of dioxiranes and related methods for site-selective C-H oxidation exhibit selectivity for remote rather than proximal 3° hydroxylation of substrates such as 1 and 2 (Figure 2), regioselectivities are often low (typically between 9:1 and 3:1 favoring remote hydroxylation). In comparison to the use of excess TFDO generated in situ our new catalytic method exhibits substantially improved selectivity for remote hydroxylation, giving ³28:1 selectivity for remote hydroxylation in the case of product 2 and complete selectivity for remote hydroxylation in the case of product 4. Solvent effects are proposed to contribute substantially to this higher selectivity.
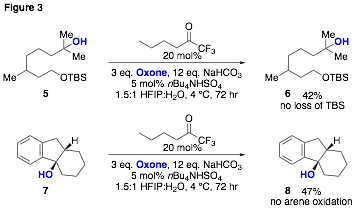
Additionally, other advantages over the use of previously developed catalytic C-H hydroxylation methods were observed for several substrates. For example, many C-H hydroxylation catalysts struggle with both acid-sensitive substrates and with selective benzylic oxidation of electron-neutral or electron-rich arenes (in the latter case arene oxidation typically predominates). As a consequence of the mild conditions developed for ketone-catalyzed hydroxylation, representative substrates having these features are tolerated (Figure 3).
In summary, our progress during this funding period has made a significant impact in that a major objective of the proposed work was accomplished. This has laid the foundation for future work on developing organocatalytic C-H hydroxylation methods that address current challenges in the field. In addition to the scientific advancement, this award has enhanced the career development of both the PI and student(s) participating in this project. For example, the PI was invited to give talks at two separate summer Gordon Research Conferences based in part on work funded by this award. The student(s) have gained valuable research training and an opportunity to publish their research.