Reports: UR756464-UR7: Unlocking the Rich Mechanochemistry of Aziridines Using Triazoline Synthons
Peter Iovine, University of San Diego
Overview. The aim of this research program is to uncover the rich mechanochemistry of aziridines and train the next generation of scientists. In year 1, we have made satisfactory progress toward our scientific goals and excellent progress on student development. ACS PRF funding supported two undergraduate research students during the summer 2017; both students have continued to conduct research in my lab and are developing new chemistries toward the development of polytriazoline polymers.
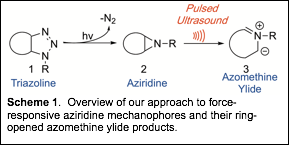
Aziridines are reactive three-member ring structures that, when embedded in a polymer main chain or side chain, function as cross-linking points. Accessing polyaziridines, however, is challenging and few examples exist. Our approach does not involve the direct synthesis of polyaziridines but rather an indirect route via the thermal or photochemical decomposition of polytriazolines. We intend to use triazolines as aziridine synthons. This approach circumvents the majority of synthetic challenges associated with synthesizing aziridines from other organic precursors and allows for a more comprehensive investigation of high density aziridine-containing polymers. Once the polyaziridines are in hand, we intend to probe how the application of mechanical force impacts the conversion of these strained three member rings into reactive intermediates (Scheme 1). We intend to mechanochemically generate azomethine ylides (structure 3, Scheme 1) from aziridine precursors (structure 2, Scheme 1) and explore productive chemistries associated with these reactive intermediates including cross-linking (strengthening) as well as heterocycle formation (increase in structural complexity).
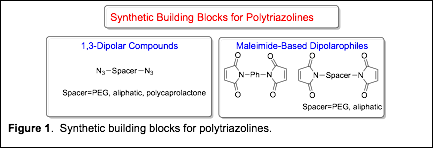
Year 1 Progress. In order to synthesize polytriazolines via a polycycloaddition type polymerization, we need high purity monomers. Triazolines are formed from the cycloaddition reaction of 1,3-dipolar compound and a dipolarophile. We focused our year 1 efforts on developing scalable syntheses of difunctional organic azides (1,3-dipolar compound) and bismaleimides (Figure 1).
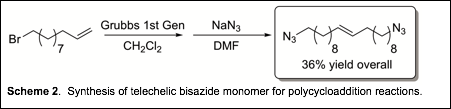 One of the challenges of synthesizing telechelic bisazides
is safety-low molecular weight bisazides are explosive. We focused on raw
materials that, when elaborated, would give a carbon:nitrogen ratio
>3. Our starting point was 11-bromo-1-undecene. Olefin metathesis
with Grubbs first generation catalyst followed by azidation resulted in a high
purity bisazide monomer in moderate yield (Scheme 2).
One of the challenges of synthesizing telechelic bisazides
is safety-low molecular weight bisazides are explosive. We focused on raw
materials that, when elaborated, would give a carbon:nitrogen ratio
>3. Our starting point was 11-bromo-1-undecene. Olefin metathesis
with Grubbs first generation catalyst followed by azidation resulted in a high
purity bisazide monomer in moderate yield (Scheme 2).
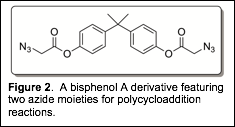
Other routes were attempted by my undergraduate students in an effort to increase the overall yield while maintaining a positive safety profile. A bisazido derivative of BPA was envisioned (Figure 2) and its synthesis attempted, however, multiple attempts failed to produce the target bisazide in meaningful quantities. This route has since been abandoned.
Having a soluble and safe telechelic bisazide in hand, we focused our attention on synthesizing a complementary bisdipolarophile that can be used in polycycloaddition polymerization. There were essentially two choices-maleimides or norbornenes. Considering we will use NMR as the main characterization tool for the final polymers, bismaleimides have some advantages over bisnorbornenes due to the simplicity of their NMR spectrum. Incorporating a flexible linker between the two maleimide units was one of our main objectives as we anticipate challenging solubility of the final polytriazoline polymers. As such, students attempted three known routes toward telechelic bismaleimides but product yields were all low.
Undergraduate Training. Two undergraduate students participated in this research during year 1. The first student, Andrew Saiz, joined my lab in the fall 2016 semester. The second student, Grayson Lang, joined the lab in the spring 2017 semester. Both students conducted research during the summer 2017 season and were paid stipends through this ACS PRF grant.
Goals for Year 2 (September 2017-August 2018). We currently have reproducible and safe synthetic routes to telechelic bisazides; these routes are delivering sufficient quantities of material such that we can move on to the polycycloaddition polymerizations. The story on the maleimide side of things is less promising. Multiple attempts to synthesize bismaleimides gave low yields and we are currently investigating more fertile routes. Commercial bismaleimides are available and we anticipate partnering these building blocks with our custom made bisazides until we can find a reliable in-house route to bismaleimides. It is our goal to have polymers made by the end of the fall 2017 semester. The spring 2018 semester can be used to characterize these novel polymers and hopefully extend the methodology to other monomers.











