Reports: DNI755562-DNI7: Synthesis and Design of Novel Metal-Doped Porous Organic Polymers: The Enhancement of Pi-Complexation with Small Unsaturated Hydrocarbons
Psaras McGrier, PhD, The Ohio State University
Covalent organic frameworks (COFs) are an exceptional class of porous crystalline materials that possess low densities, great thermal stabilities, and high internal surface areas. While the construction of two-dimensional (2D) COFs containing metalated porphyrin, phthalocyanine, and bipyridine units have become fairly prevalent, finding efficient ways to synthesize novel three-dimensional (3D) COFs that can be metalated without compromising the surface area or pore volume of the material is still a challenge. As a consequence, only one metalated 3D COF has been reported. The limited number of metalated 3D COFs is likely attributed to the synthetic challenge of utilizing the right geometrically shaped monomers that can not only produce crystalline polymeric networks but also form strong complexes with metal ions.
Herein, we report the synthesis and metalation of a mesoporous 3D COF containing C3-symmetric pi-electron conjugated dehydrobenzoannulene (DBA) and tetrahedral tetra(4-dihydroxyborylphenyl)methane (TBPM) units. DBAs are planar triangular shaped macrocycles that are capable of forming strong metal complexes with Li, and Ca, and low oxidation state transition metals. The planarity and metal binding properties of DBAs are comparable to porphyrin and phthalocyanine ligands, which typically use hard nitrogen atoms to bind metals. In contrast, DBAs are neutral compounds that contain soft ligands capable of donating 2-4 electrons per alkyne depending on the electronic demands of the metals. We report that DBA[12] and TBPM monomers can be utilized to form a crystalline 3D polymer network. Interestingly, both materials display great uptake capacities for ethane and ethylene gas.
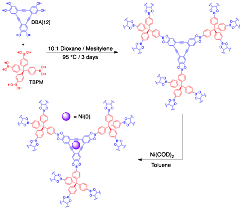
Scheme 1. Synthesis of DBA-3D-COF 1 and Ni-DBA-3D-COF
DBA-3D-COF 1 was synthesized under solvothermal conditions by reacting DBA[12] with TBPM in a 10:1 (v/v) 1,4-dioxane and mesitylene mixture in flame-sealed glass ampules at 95 C for 3 days (Scheme 1). DBA-3D-COF 1 was obtained by filtration and washed with acetonitrile in an argon-purged glove box to produce a light green crystalline powder. The ideal reaction conditions were obtained by screening different temperatures, monomer and solvent ratios, and reaction times. It should be noted that samples in which both DBA[12] and TBPM monomers were fully soluble prior to sealing the ampules typically yielded the best results. Thermogravimetric analysis revealed that DBA-3D-COF 1 maintains ~ 95% of its weight up to 458 C. Scanning electron microscopy (SEM) images revealed the formation of rhombic shaped crystallites. Metalation was achieved by adding a solution containing 10 wt% Ni(COD)2 dissolved in 1 mL of dry toluene to ~34 mg of DBA-3D-COF 1. The mixture was then stirred at room temperature in an argon-purged glove box for 24 h to produce Ni-DBA-3D-COF as a dark purple crystalline powder. Ni-DBA-3D-COF was obtained by filtration, washed with dry toluene to remove excess Ni(COD)2, and dried under vacuum at 35 C for 16 h. The 3D COFs were characterized by Fourier transform infrared (FT-IR) spectroscopy, 13C cross-polarization magic angle spinning (CP-MAS) NMR, and powder X-ray diffraction (PXRD).
The porosity of DBA-3D-COF 1 and Ni-DBA-3D-COF were determined by nitrogen gas adsorption measurements at 77 K. DBA-3D-COF 1 exhibited a type-IV isotherm displaying a sharp uptake at low relative pressure (P/P0 < 0.1) followed by a sharp step between P/P0 = 0.05 and 0.13 which is indicative of a mesoporous material. The Brunauer-Emmet-Teller (BET) model was applied over the low-pressure region (0.07 < P/P0 < 0.11) of the isotherm to provide a surface area of 5083 m2 g-1. Ni-DBA-3D-COF also exhibited a type-IV isotherm. Application of the BET model over the low-pressure 0.07 < P/P0 < 0.11 range provided a surface area of 4763 m2 g-1.
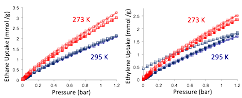
Figure 1. Ethane (left) and ethylene (right) adsorption/desorption isotherms for DBA-3D-COF 1 (circle) and Ni-DBA-3D-COF (square).
To determine if Ni-DBA-3D-COF could be effective at performing ethane/ethylene separations, we measured gas adsorption isotherms for both COFs at 273 and 295 K from 0 to 1.2 bar (Figure 1). DBA-3D-COF 1 exhibited swift uptake of ethane at low pressures reaching capacities of 3.24 mmol g-1 at 273 K and 2.09 mmol g-1 at 295 K. The ethylene uptake capacities were much lower reaching values of 2.52 mmol g-1 at 273 K and 1.70 mmol g-1 at 295 K. In comparison, Ni-DBA-3D-COF displayed uptake capacities of 3.01 mmol g-1 and 2.16 mmol g-1 for ethane, and 2.36 mmol g-1 and 1.83 mmol g-1 for ethylene at 273 and 295 K, respectively. Surprisingly, the metalated Ni-DBA-3D-COF displayed only a modest increase of 0.07 mmol for ethane, and 0.13 mmol for ethylene at 295 K. These slight increases are likely attributed to the ability of the open Ni(0) sites to polarize ethane, and weak pi-complexation between the d-orbitals of Ni(0) and the pi orbitals of ethylene. The ethane/ethylene selectivities of DBA-3D-COF 1 and Ni-DBA-3D-COF were calculated using initial slope calculations in the pressure range of 0-0.1 bar to afford values of 1.25 and 1.28 at 273 K, and 1.24 and 1.15 at 295 K, respectively. The isosteric heats of adsorption (Qst) were estimated using the virial method to assess the binding affinity of ethane and ethylene to both materials. DBA-3D-COF 1 exhibited Qst values of 16.8 kJ mol-1 for ethane and 15.9 kJ mol-1 ethylene, while Ni-DBA-3D-COF displayed Qst values of 11.6 kJ mol-1 for ethane and 9.7 kJ mol-1 for ethylene at 295 K, at zero coverage.
In conclusion, we have demonstrated that a DBA[12] monomer can be used to create a highly porous 3D COF that retains its crystallinity upon metalation with Ni. Metalation of DBA-3D-COF 1 results in a minimal reduction in the surface area and pore volume of the material. The proof-of-principle is important, as DBA monomers also have the ability to bind Li, Ca, and other low oxidation state transition metals. This research puts the PI and the graduate students involved with the project in a position to utilize metalated DBA-based COFs for applications related to catalysis, gas storage, spintronics, and separations.