Reports: DNI1053678-DNI10: New Strategies to Synthesize Covalent Organic Frameworks
Jian Zhang, University of Nebraska, Lincoln
The goal of this project was to develop new methods for the synthesis of covalent organic frameworks and porous organic frameworks. In the first year of this grant, we have developed a facile synthetic approach to access carbazolic porous organic frameworks (Cz-POFs) and further explored their visible light photoredox catalytic activities. In the second year, we developed two different approaches to prepare Cz-POFs with tunable photoredox properties and evaluated their applications in organic synthesis. In the third year, we had the opportunity to extend to small organic molecules for photoredox catalysis.
1. Cz-POFs constructed by copolymerization
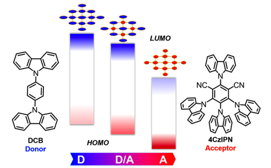
One versatile approach to tune the optoelectronic properties of Cz-POFs is to change the ratio of donor and acceptor D-A Cz-POF via co-polymerization. We select two carbazole-based monomers, DCB (1,4-bis(9H-carbazol-9-yl)benzene) and 4CzIPN (1,2,3,5-tetrakis(carbazol-9-yl)-4,6-dicyanobenzene), as comonomers because the carbazole moieties can undergo a smooth and uniform FeCl3-induced oxidative polymerization to form carbazole-based POFs (Cz-POFs).
Fig. 1. Schematic Diagram of the Design of Copolymer CzCPs
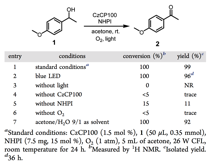
We first tested CzCP100 for the oxidation of a prototypic benzyl alcohol, 1-(4-methoxyphenyl)ethan-1-ol. The CzCP100/NHPI/O2 photocatalytic system indeed exhibited an excellent activity and selectivity. A blue light-emitting diode (LED) was also an effective light source, although a longer reaction time (36 h) was required. Control experiments confirmed the essential role of photocatalyst, NHPI, light, and O2. A reduced loading of NHPI (10 mol %) gave a lower yield (70%), while a higher loading (20 mol %) led to a lower selectivity (100% conversion with 81% yield). The catalytic system also displayed a good tolerance to H2O: a 92% yield was obtained in a mixture of acetone and water (9/1 v/v) (Table 2, entry 7). Overall, CzCPs showed good to excellent activities for the aerobic oxidation of benzyl alcohol 1 (47−99% yield) with excellent selectivity (the difference between conversion and yield is less than 5%).
Table 1. Initial Studies for the Photoinduced Oxidation of Benzylic Alcohol 1
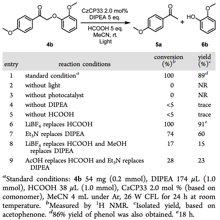
As for the reduction cleavage, in the presence of a Brønsted acid, a sacrificial electron donor, and light irradiation from a 26 W white CFL at room temperature, lignin oxide 4b was efficiently converted to acetophenone and phenol. Control experiments confirmed that the photocatalyst, formic acid, DIPEA, and light are all essential for this reaction (Table 2, entries 2−5). Lewis acid LiBF4 also promotes this reaction. It is noteworthy that the reaction is significantly retarded in the presence of a weaker Brønsted acid (i.e., acetic acid) or a weaker hydrogen donor (i.e., Et3N and MeOH). In comparison to CzCP33, other CzCPs are less active under the same reaction conditions (21−81% yield).
Table 2. Initial Studies for the Photocatalytic Cleavage of Lignin β-O-4 Ketones
Overall, we have demonstrated that the copolymerization of carbazolic electron donor and electron acceptor based comonomers is an effective strategy to fine tune the photoredox potentials of the resulting π-conjugated CzCPs. These CzCPs can be used as excellent heterogeneous photocatalysts for degradation of the lignin β-O-4 models. CzCPs are highly stable and can be recycled multiple times without a significant loss of the catalytic activity.
2. POFs Pyrene-Based Fluorophores–“Inner Sphere” Electron Transfer.
With the support of ACS-PRF, we recently stated a project aims to test if inner-sphere electron transfer can be utilized in photoredox catalysis. “Inner-sphere” ET occurs adiabatically within an electron donor-acceptor (EDA) complex, where the strong electronic coupling between electron donor and acceptor circumvents the crossing of high potential energy surface, and thus proceeds significantly faster than that predicted by the “outer-sphere” model.
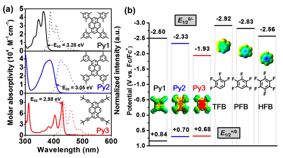
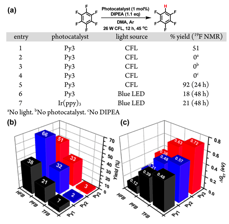
Here, we chose three highly fluorescent pyrene derivatives Py1, Py2, and Py3, as the photocatalysts and three polyfluoroarenes as the substrates (Fig. 2). The three Py photocatalysts provide a systematic variation of steric hindrance. Py2 and Py3 also appreciably absorb visible light (λ > 390 nm, Fig. 2a). According to the outer-sphere model, ΔGET was calculated ranging from 0.12 to 0.72 eV. We envisioned that, however, upon the formation of the “π-hole−π” Py:FA complexes, a good molecular orbital overlap could become possible to facilitate the inner-sphere ET to form FA•−. Followed by the expulsion of fluoride and hydrogen atom abstraction, an HDF process of FA should be developed.
Fig. 2. (a) UV−vis absorption and fluorescence emission spectra (λex = 360 nm) of Py in DMA (dimethylacetamide). (b) Reduction potentials (all potentials mentioned hereafter are against Fc+/Fc0, Fc = ferrocene) and density functional theory (DFT) computed electrostatic surface potential maps (inset) of Py and FA.
Indeed, despite of the lack of charge-transfer interaction in the pyrene/fluoroarene complex and a sizable underpotential, local excitation of pyrene efficiently promotes the injection of electron into fluoroarene thanks to the close contact. Overall, the four Py:FA pairs with relatively large Kc gave the HDF product in good yields (12 h, 32−66%) (Fig. 3b.).
Fig. 3. (a) HDF reaction for Py3:HFB. (b) HDF yields and (c) ΔGET for nine Py:FA pairs under the reaction conditions shown in Figure 3a.
Overall, we have described a new example showing the inner-sphere ET between photocatalyst and substrate plays an important role in the overall photocatalytic reaction. The appreciable formation constant and favorable steric hindrance within the “π-hole−π” Py-FA complexes facilitate an inner-sphere ET despite unfavorable ET energetics. This process is utilized in the hydrodefluorination reaction to access partially fluorinated arenes. Our work points to the further development of the design paradigm for photoredox catalysis where the size and shape of photocatalyst can be fine-tuned to enhance the overall catalytic activity.
Summary.
Funding for this project from the past three years has allowed us to develop new synthetic strategies for functional porous organic frameworks as well as new organic fluorophores and explore their novel applications in photoredox catalysis for organic transformations. The newly developed D-A Cz-POFs based heterogeneous solid exhibited enhanced photocatalytic activities. Moreover, this grant also facilitated a new direction in my group to exploit the new intermolecular interaction such as pi-pi interaction and hydrogen bond in photoredox catalysis. These result will be used to pursue funding from NSF.