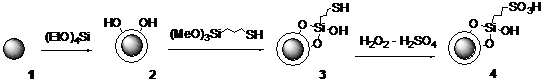Reports: UR555931-UR5: Room Temperature Friedel-Crafts Alkylation Using Magnetic Nanoparticle Catalysts in Oscillating Magnetic Fields
Ananda Amarasekara, Ph.D, Prairie View A&M University
The goal of this project is to study a new type of a heterogeneous catalyst activation method. To test the new idea a series of -SO3H group attached silica coated magnetic CoFe2O4 nanoparticles are prepared and used as catalysts for Friedel-Crafts alkylation under the influence of 20-500 Hz oscillating magnetic fields.
In the initial phase of this study n-propyl sulfonic acid group bearing silica coated magnetic nanoparticles (MNPSiSO3H) (4) were prepared by following the literature procedure1,2 as shown in figure 1.
Figure 1. Synthesis of sulfonic acid bearing silica coated magnetic nanoparticles (MNPSiSO3H) (4)
The magnetic core (1) of the nanoparticle catalysts were first prepared by micelle-template method using CoCl2.6H2O, FeCl2.4H2O, and sodium dodecyl sulfate in the presence of methylamine. Then the nanoparticles were covered with a silica shell by treatment with tetraethyl orthosilicate (TEOS) to produce silica coated nanoparticles (2, MNPSi). In the next step (3-mercaptopropyl)trimethoxysilane was added to the MNPSi dispersed in i-PrOH in the presence of aqueous NH3, under sonication to attach the mercaptopropyl group. Finally, silica-coated magnetic nanoparticles functionalized with propylsulfonic acid groups (4, MNPSiSO3H) were prepared by oxidation of thiol groups by using 30% aqueous H2O2-H2SO4 mixture. Sulfonic acid group loading in the catalyst was measured as 0.48 mmol/g by titration with aq. NaOH, using phenolphthalein as the indicator.
We have studied the alkylation experiment of phenol using t-butanol under oscillating magnetic fields generated by two different methods. The first method involves the electromagnetically generated oscillating field using a 13 mm (d)X 30 mm (l), 600 turn solenoid powered by a 25W amplifier (home built using TDA2050 IC). The sine wave AC input to the amplifier was generated using software: Visual Analyzer 2011XE Beta 0.3.2. The second method involves mechanical rotation of a CMS Magnetics N45 Neodymium cube magnet (3.81 cm X 3.81 cm X 2.54 cm) using a RS-445PA-14230 24 V DC motor coupled via a 4:1 gear system.
In experiments using the solenoid generated oscillating magnetic fields a phenol : t-butanol = 1 : 3 mole mixture with MNPSiSO3H (2 mol%) in a glass vial was placed inside the solenoid. This experiment produced a 8% yield of a mixture of alkylated phenols after 24 hr. The temperature inside the reaction mixture rose up to about 70 °C during the experiment due to the solenoid in runs longer than 30 minutes and the temperature increase was particularly significant at frequencies higher than 100 Hz. Since the thermal effect itself can produce the catalyst activation it is not clear whether the catalyst works due to heat or due to the oscillating magnetic field. Therefore, we have looked at the second method of generating the magnetic field as well. In addition, we are currently working on sample cooling methods to eliminate the thermal effect. In the second method we have used the mechanical rotation of a strong permanent magnet to generate the oscillating magnetic field as shown in Figure 2.
Figure 2. Experimental setup used for creating an oscillating magnetic field by mechanical rotation of a strong Neodymium magnet.
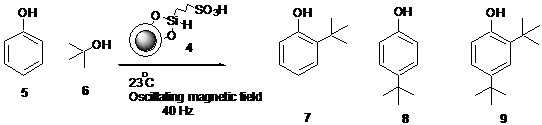
Figure 3. Alkylation of phenol with t-BuOH at 23 °C, using magnetic nanoparticle catalyst MNPSiSO3H (4) under oscillating magnetic field created by the mechanical rotation of a strong Neodymium magnet.
The reaction vial with phenol : t-butanol = 1 : 3 mole mixture and MNPSiSO3H (2 mol%) was placed ~ 1 mm from the rotating magnet and the speed of the motor was controlled using a DC power regulator to give a 40 Hz oscillating field. The temperature inside the solution remained close to room temperature in equilibrium throughout the reaction period. At the end of the reaction period, magnetic catalyst was removed by a strong neodymium permanent magnet and the product was analyzed by GC and 1H NMR.
Table 1. Comparison of the catalytic activity of sulfonic acid group functionalized silica coated magnetic nanoparticles (4, MNPSiSO3H) in the alkylation of phenol with t-BuOH at 23 °C, using oscillating field generated by rotation of a CMS Magnetics N45 Neodymium cube magnet at 40 Hz
Reaction condition |
Time (h) |
% yield of alkylated phenols |
Product composition 7 : 8 : 9 |
23 °C, no magnetic field |
24.0 |
0 |
- |
80 °C, no magnetic field |
24.0 |
6 ±1 |
2 : 2 : 5 |
40 Hz oscillating magnetic field, 23 °C |
8.0 |
11 ±2 |
2 : 1 : 5 |
40 Hz oscillating magnetic field, 23 °C |
24.0 |
15 ±3 |
2 : 1 : 5 |
phenol : t-butanol = 1 : 3 mole mixture with MNPSiSO3H (2 mol%) were used in all experiments
The catalytic activity and alkylation yields seen in this initial study is small, but we have clearly shown that oscillating magnetic field can be used to activate the catalytic activity of an acidic group attached to the surface of a magnetic nano particle. In addition to the first catalyst we prepared by following a literature procedure, we are currently working on new methods for immobilization of the sulfonic acid catalyst function using imidazolium ionic liquid core groups and sultones as described in the grant proposal. We are expecting to achieve better alkylation yields with new -SO3H functionalized magnetic nanoparticle catalysts. This type of 100% recyclable room temperature catalysts can revolutionize the catalysis science research, and can pave the way to a new generation of catalysts useful in petrochemical field and many other areas.
Involvement of undergraduate students in research
Two Prairie View A&M undergraduate students worked part-time on the project during the regular semesters in 2016-17 reporting period. The first student, a chemistry-biology major is a sophomore and continues to work on the project. The second student, a senior, graduated with B.S. in chemistry in spring 2017, was accepted to a graduate program from fall 2017 and currently studying for her Ph.D. in chemistry.
References
1. Gill, C. S.; Price, B. A.; Jones, C. W., J. Catal., 2007, 251 (1), 145-152.
2. Takagaki, A.; Nishimura, M.; Nishimura, S.; K, E., Chem. Lett., 2011, 40, 1195-1197.