Reports: ND655842-ND6: Understanding the Photoluminescence Mechanism of Water Soluble Polymer Clusters for Petroleum Exploration
Liangfeng Sun, PhD, Bowling Green State University
Overview
The goal of the proposed research is to find out the light-emitting mechanism of water-soluble polymer clusters for petroleum exploration. In the past year, two new methods – photoluminescence excitation spectroscopy and time-resolved emission spectroscopy – were used to investigate the emission mechanism of the polymer cluster, in addition to the methods used earlier such as time-resolved photoluminescent and photoluminescence quantum yield. Based on the new data and the data obtained earlier, we conclude that excitation dependent fluorescence may not be caused by the solvent relaxation but by the different emitting centers. Some of the results have been published in MRS Advances – a peer-reviewed journal published by Cambridge Core.
The ACS-PRF grant is the first grant the PI obtained before tenure. The research supported by this grant made a significant positive impact on his tenure process. The interesting results obtained so far have opened the door to more advanced research on the polymer clusters. The PI is currently preparing a proposal on the photophysics of polymer clusters to be submitted to NSF. In the past two years, this grant has supported three undergraduate students, two MS-degree graduate students and one PhD-degree graduate students to do the research on polymer clusters, and received training on a variety of research methods and instruments. Among the six students, four of them has obtained their corresponding degrees and graduated.
The main results obtained in the past year are listed below.
Result 1: Electron microscopy
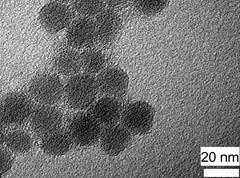
The method of synthesis of C dots is adapted from a previously reported method with some modification.[1] The polymer clusters exhibit quite a uniform size under the electron microscope (Figure 1). No lattice structure was observed from the polymer clusters. They are basically amorphous.
Figure 1. The transmission electron microscope (TEM) image of polymer clusters.
Result 2: Optical absorption and photoluminescence
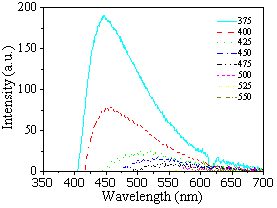
A remarkable optical property of polymer clusters is the excitation-dependent fluorescence, which violates the Kasha-Vavilov rule. In Figure 2, the fluorescence spectra of polymer clusters show two different components: the excitation-independent component in the excitation wavelength from 275 nm to 400 nm, and the excitation-dependent component in the excitation wavelength from 425 nm to 550 nm, which clearly indicates the two different photoluminescence mechanisms. For the independent part, the photoluminescence of polymer clusters residents around 450 nm. In the previous work,[1] the dual emission mechanism was attributed to the presence of organic fluorophores for the excitation-independent range and carbogenic cores for the excitation-dependent range.
Figure 2. Excitation independent (Left) and dependent (Right) photoluminescence spectra of PVA (Polyvinyl alcohol) dispersions of polymer clusters.
Results 3: Absolute photoluminescence quantum yield
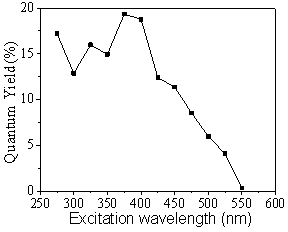
As shown in Figure 3, the quantum yield of polymer clusters varies with the excitation wavelength. The quantum yield generally shows two stages with the increase of excitation wavelength: when excited from 275nm to 400nm, the quantum yield of polymer clusters fluctuates between 12.8% and 19.3%; while for the range of 425nm to 550nm, the quantum yield keeps decreasing from 18.8% to 0.34%, which also indicates the existence of two different photoluminescence mechanisms.
Figure 3. Excitation dependent photoluminescence quantum yield of polymer clusters.
Results 4: Photoluminescence lifetime
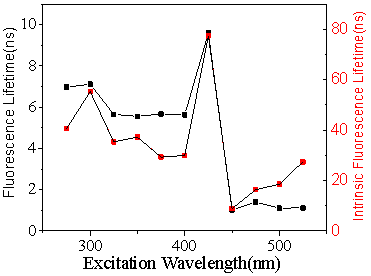
The photoluminescence lifetime of the polymer clusters dispersed in PVA depends on the excitation wavelength (Figure 4). It shows two different stages: the 1st stage is around 6 ns with excitation wavelength from 275 nm to 400 nm, the 2nd stage is around 1 ns. The radiative lifetime was calculated through dividing the photoluminescence lifetime by quantum yield. It shows two stages too. For the photoluminescence excitation-independent part, the radiative lifetime shows a continuous declining trend from 55 ns to 30 ns. However, after a sudden rising to 78ns, the radiative lifetime falls to 9 ns with a slightly growing trend from 9 ns to 28 ns. The step-function-like change of the lifetime also indicates there are two different emission states. It is consistent with the result obtained from the photoluminescence spectroscopy that shows the PL spectrum is excitation-wavelength dependent when the excitation wavelength is longer than 425 nm, but is excitation-wavelength independent when the excitation wavelength is shorter.
Figure 4. Excitation dependent photoluminescence lifetime of PVA (Polyvinyl alcohol) dispersions of polymer clusters.
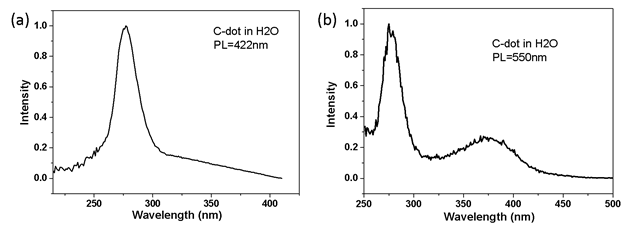
Results 5: Photoluminescence excitation spectroscopy
Photoluminescence excitation (PLE) spectroscopy shows the spectrum similar to an absorption spectrum. More than that, it can probe the energy structure of the polymer clusters emit at a certain wavelength. Our results show that there is only one peak in the PLE spectrum for the emission at 422 nm, but two peaks for the emission at 550 nm. This reveals that there are two different emission mechanisms: one is responsible for the emission around 422 nm and the other for the emission at 550 nm.
Figure 5. Photoluminescence excitation spectra of polymer clusters at (a) 422 nm and (b) 550 nm.
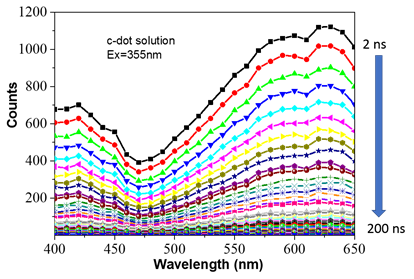
Results 6: Time-resolved emission spectra
Figure 6. Time-resolved emission spectra of polymer clusters dispersed in water. The spectra were taken from 2 ns after excitation until 200 ns with a time step of 2 ns.
The spectra from the polymer clusters dispersed in water were taken at time 2 ns after pulsed laser excitation (excitation wavelength 355 nm) until 200 ns. The time-resolved emission spectra can reveal the effect of solvent relaxation on the spectrum shift of the sample.[2] However, our data show no shift of the spectrum peak except the decrease of the intensity (Figure 6). These results tell us that either the solvent relaxation has no effect on the polymer-clusters spectrum or the relaxation time is much longer than the fluorescence lifetime.
References
[1] M. J. Krysmann, A. Kelarakis, P. Dallas, E. P. Giannelis, J. Am. Chem. Soc. 2012, 134, 747.
[2] S. Khan, A. Gupta, N. C. Verma, C. K. Nandi, Nano Lett. 2015.