Reports: ND254853-ND2: Formation and Early Diagenesis of Microbialites in Storr's Lake, San Salvador, Bahamas: An Alkaline Earth Metal Isotope Investigation
Elizabeth M. Griffith, PhD, The Ohio State University
David J. Wronkiewicz, PhD, Missouri University of Science and Technology
Accomplishments during the second year of the funded proposal include completion of the M.S. program by one student at UT Arlington, method development, and analysis of magnesium isotopes at the Metal Isotope Laboratory at Penn State University in collaboration with Dr. Matt Fantle. An undergraduate student from Missouri University of Science & Technology worked with Dr. David Wronkiewicz and a second M.S. student at UT Arlington continued work on her M.S. degree analyzing some of the data and samples from the field trip in January 2016. The grant will be moving to The Ohio State University with the PI who accepted a faculty position there starting August 2017.
The formation of impressive modern irregular, hard microbialite mounds have previously been identified and studied in the turbid and seasonally hypersaline Storr’s Lake on San Salvador Island in the Bahamas (Figure 1). The high turbidity (and light reddish-brown color) of the shallow lake (<2 m) is derived entirely from microorganisms; both as living planktonic, motile forms, and from dead organisms suspended in the water leading to rapid attenuation of sunlight in the water (Figure 2).
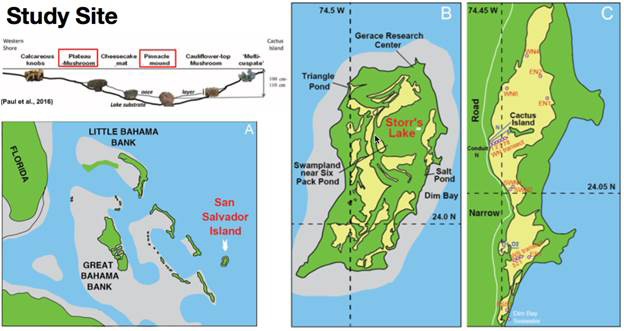 |
Figure 1 – Location map of (A) The Bahamas, (B) San Salvador Island with the location of Storr’s Lake, and (C) sampling locations and transects (labeled in red) of Storr’s Lake for this study. Transect N, P, D1, and D2 were published in Neumann et al. (1989), Paul et al. (2016), and Dupraz et al. (2013), respectively. Upper left-hand panel highlights the general position and relative depth of stromatolites across the transect from Paul et al. (2016) with red boxes around the two types of stromatolitic mounds analyzed.
Figure 2 – Photograph taken
at Storr’s Lake on San Salvador Island looking to the
west from the eastern shore in January 2016. Note the high turbidity and
reddish-brown
color of then lake water and white foam along the shore that forms by wind agitating the water rich in dissolved organic matter.
Two stromatoltic mounds collected in January 2016 were analyzed in detail as described here. Both mounds were collected within the deep part of the lake (water depth was greater than 110 cm at the time of collection). One of the samples chosen was buried in soft organic-rich calcareous ooze, but the other was not. Lake water was supersaturated with respect to calcite and aragonite (and dolomite) and higher saturation state than seawater. Lake water had conductivity values about 1.2 x seawater and pH ~8.4. Major cations show little variation with depth.
Precipitation of carbonate minerals in stromatolites is generally considered to be an organomineralization process forming laminae. Organomineralization is induced by metabolic activities of microbes – through production and degradation of extracellular polymeric substance (EPS) and processes that modify pH and alkalinity. Carbon isotopic analysis can give some insight into processes influencing organomineralization. For our two samples investigated, it appears that both photosynthesis (evidenced by high carbon isotope values) and EPS degradation (evidenced by low carbon isotope values) control organomineralization (data not shown). This agrees with previous data from Paul et al. (2016), which found both phototropic and heterotrophic bacterial taxa.
Variations in mineralogy (high-Mg calcite and aragonite) have been ascribed to early diagenesis and authigenic mineral formation and/or changes in lake water chemistry. Both mounds show a distinct trend of >70% high-Mg calcite in the top irregular surface and >70% aragonite in the interior with almost perfect correlation between mineralogy and Mg/Ca and Sr/Ca ratios measured for the carbonate layers (n = 18; Figures 3-4).
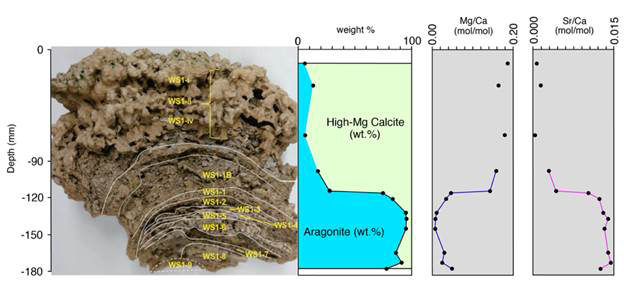 |
Figure 3 – Sampling locations, mineralogical compositions from XRD and Mg/Ca molar ratios and Sr/Ca molar ratios from ICP-OES of the dissolved solid subsamples of the WS1 pinnacle mound sample. Lines connect samples that were sampled in a continuous sequence.
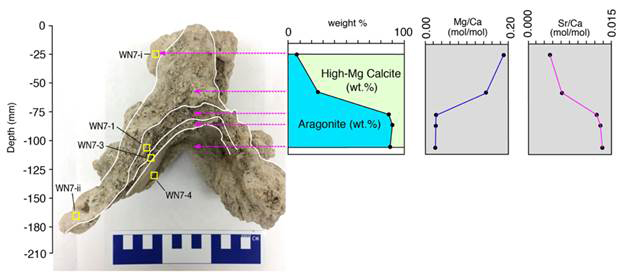 |
Figure 4 – Sampling locations, mineralogical compositions from XRD and Mg/Ca molar ratios and Sr/Ca molar ratios from ICP-OES of the dissolved solid subsamples of the WN7 plateau-mushroom sample (below).
Methods were refined for sample preparation in the clean lab at UT Arlington and then analyzed for their Mg isotopic compositions on the multi collector inductively coupled plasma mass spectrometer (MC-ICP-MS) in Penn State’s Metal Isotope Laboratory using either the Aridus or Apex desolvating systems. Two columns were deemed necessary to completely separate Mg from Ca and other cations in lake waters and dissolved stromatolite samples. An example of the elution curves of Mg and matrix elements for a seawater sample is shown in Figure 5 for the second column procedure. After initial concerns during isotope analysis it was thought to be necessary to clean the cation exchange resin on the columns with HF, ultrapure water, and HCl prior to conditioning in preparation for sample processing. Since this was the first time these types of samples were prepared for analysis in the lab, much time was spent developing and confirming these methods.
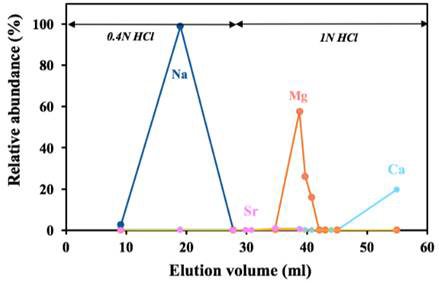 |
Figure 5 – Elution curves of Mg and matrix elements for a seawater sample for the second column chromatography necessary for similar lake water samples.
Initial results indicate that seawater is probably the primary source for Storr’s Lake water and the two stromatolite mound samples have overall similar Mg isotopic compositions (Figure 6), with the aragonitic layers ~0.2 permil heavier than the high-Mg calcite layers (Figure 7). However, it is estimated that >80% of the Mg measured in these layers is coming from high-Mg calcite and not aragonite. The isotopic offset between lake water and the stromatolites ranges from -1.5 to -2.2 permil, similar to marine biogenic carbonates of high-Mg calcite and aragonite previously reported (Figure 6). No clear trend exists between magnesium and carbon or oxygen isotopes.
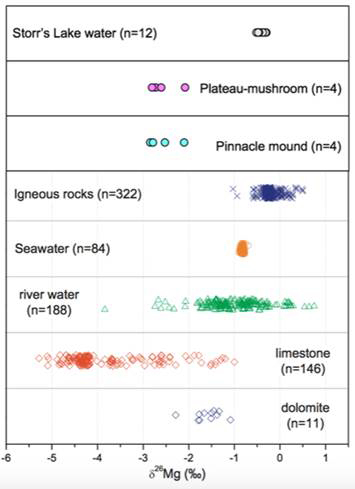 |
Figure 6 – Comparison of δ26Mg values of Storr’s Lake water and stromatolitic sublayers with published Mg isotope data from different geological reservoirs (modified from Li et al., 2012).
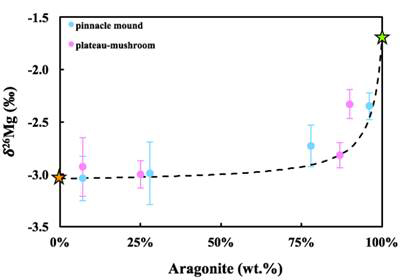 |
Figure 7 – Comparison of δ26Mg values of Storr’s Lake stromatolitic sublayers with the aragonite weight percent determined by XRD.











