Reports: ND656216-ND6: Measuring Carbon Dioxide Dynamics in Metal-Organic Frameworks on the Time Scale of Computer Simulations
Aaron M. Massari, University of Minnesota, Twin Cities
The goal of this project is to use two-dimensional IR spectroscopy (2D-IR) to study adsorbed CO2 inside of MOF materials to monitor the fast configurational rearrangements during the course of an adsorption isotherm. Using this methodology, we will ultimately study a series of isostructural MOFs with different open metal binding sites for CO2 to determine the effect of the metal identity on time dependent structural motions of the adsorbed gas. We will also study a set of structurally similar MOFs that offer varying degrees of flexibility during gas adsorption to determine the impact of framework mobility on CO2 configurational dynamics, and will expand this work to also investigate the influence of hydration. These studies will generate experimental data in the form of frequency-frequency correlation functions (FFCF) that characterize the molecular dynamics and are directly comparable to FFCFs obtained from simulations.
During this funding period, progress was intermittent due to a lack of personnel for the project. However, the activities that were supported have moved the project forward to a point that we are ready to make significant progress in the upcoming year. These results are listed below.
First, a measurement platform was developed upon which to synthesize free-standing MOF materials that will adsorb CO2 and also be amenable to 2D-IR spectroscopy. Commercially available anodic aluminum oxide (AAO) membranes were used as the MOF substrate and a sacrificial aluminum source. Expanding on the work of Zhang and coworkers,1 AAO membranes were reacted with 1,4-benzenedicarboxylic acid in water under high pressure. This resulted in the growth of MIL-53 MOF crystallites on the surface of the AAO discs. Scanning electron microscopy (SEM) was used to characterize the growth progress as shown in Figure 1. The initial AAO pore sizes were 100-200 nm in diameter, and the resulting polycrystalline films produced thick coatings of MIL-53 after 18-24 hours of reacting under high pressure.
Figure 1. AAO membranes after reacting with 1,4-benzenedicarboxylic acid under high pressure for a) 0 hrs, b) 6 hrs, c) 18 hrs.
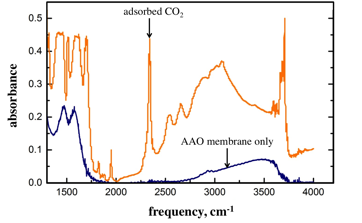
MIL-53 is made up of chains of AlO4(OH)2 octahedra interconnected by 1,4-benzene-dicarboxylate groups.2 This MOF serves the goals of this proposed study in several ways. Most importantly, it binds CO2 and has been studied for its gas sorption properties. In addition, it forms continuous free-standing films on AAO membranes, which are conveniently IR transparent and thermally robust to withstand the conditions required for MOF activation. Growing MIL-53 on the AAO membrane is apparent by FTIR spectroscopy. Figure 2 shows the FTIR spectrum of a representative sample of MIL-53 grown on an AAO membrane. Most importantly, we have been able to consistently activate the MOF and bind CO2 gas. The FTIR spectrum (Figure 2) shows the characteristic adsorbed CO2 asymmetric stretching vibration on the low frequency side of gas-phase CO2. By controlling the dosing of the sample with automated flow controllers we were able to adsorb a range of concentrations of CO2 to this MOF. Nitrogen adsorption isotherms were carried out to determine the surface area of the prepared MOF films, revealing that the area was high after significant soxhlet extraction to remove unreacted ligand.
Figure 2. FTIR spectra of AAO membrane without MOF (blue) and after 24 hours of MIL-53 growth followed by activation at 10 Torr and 200 °C (orange).
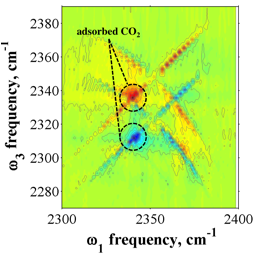
In parallel with this work characterizing MOF growth and behavior, we used 2D-IR spectroscopy to study CO2 adsorbed on the inside of a polydimethylsiloxane (PDMS) membrane. This sample served as a proxy to MOF samples while the details of MOF preparation were developed, as described above. Adsorption of CO2 in PDMS is non-specific but nonetheless gave a characteristic FTIR peak that could be studied by 2D-IR. The first 2D-IR spectrum of this sample ever recorded (to the best of our knowledge) is shown in Figure 3. The unusual "X" patterned features arise from gas-phase CO2 within the film (an unexpected result) and the round red-shifted feature is the non-specifically adsorbed CO2 gas within the polymer. Moving forward, the pieces to complete the proposed work are in place and they will be put together in the upcoming funding period.
Figure 2. 2D-IR spectrum of CO2 inside a PDMS membrane. Positive and negative-going X-shaped features correspond to gas-phase CO2 within the polymer. The round features marked with dashed lines are adsorbed CO2 as will be monitored within the MOF structure.
References
1. Zhang, Y. L.; Gao, Q. M.; Lin, Z.; Zhang, T.; Xu, J. D.; Tan, Y. L.; Tian, W. Q.; Jiang, L., Constructing Free Standing Metal Organic Framework MIL-53 Membrane Based on Anodized Aluminum Oxide Precursor. Sci. Rep. 2014, 4.
2. Loiseau, T.; Serre, C.; Huguenard, C.; Fink, G.; Taulelle, F.; Henry, M.; Bataille, T.; Ferey, G., A rationale for the large breathing of the porous aluminum terephthalate (MIL-53) upon hydration. Chem. Eur. J. 2004, 10 (6), 1373-1382.