Reports: ND656803-ND6: Computer Modeling of Threaded Surfactant Aggregates
Elena E. Dormidontova, PhD, University of Connecticut
The objective of this new direction (ND) project is to investigate using computer modeling the possibility of threading of wormlike micelles formed by synthetic surfactants by polymers of different hydrophobicity and analyze how it would affect (viscoelastic) properties of wormlike micelles and conformation of embedded polymer. As wormlike micelles are actively used in oil recovery threading wormlike micelles by polymers may enhance their stability, the hypothesis that will be tested in the course of this project. Computer modeling results will be tested experimentally by collaborator’s group of Prof. Philippova. Being a new direction this project will help the PI and involved graduate student to gain experience in the new area of surfactant self-assembly.
Polymer encapsulation in potassium oleate wormlike micelles.
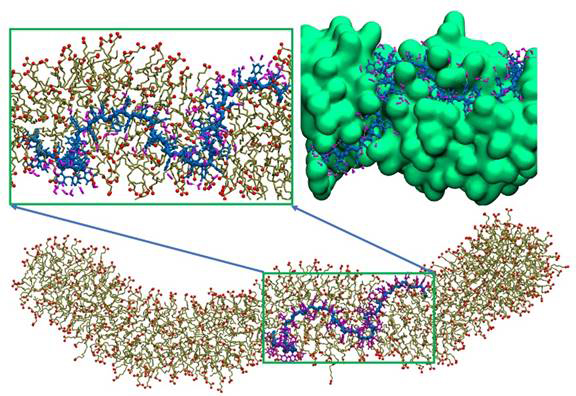
Using united atom molecular dynamics simulations with the GROMOS 53a6 force field we studied interactions of wormlike micelle (WLM) formed by the anionic surfactant potassium oleate in the presence of 6 wt % potassium chloride (KCl) and an uncharged polymer - poly(4-vinylpyridine) (P4VP). We investigated under what conditions the stable complexation between the polymer and surfactant can be achieved and analyzed the polymer conformation and location in the polymer/surfactant complex. NPT simulations were performed using the GPU version of GROMACS 4.6.5 at 1 bar pressure (compressibility 4.5 x10-5 bar -1) and temperature 300 K. We find that P4VP is solubilized by the wormlike micelles at the interface between the tails and head groups of surfactant, thus screening the hydrophobic polymer backbone from interactions with water while maintaining hydrogen bonding between the pyridine rings and water, as shown in Figure 1. The micelles provide a thermodynamically favored environment for the macromolecules, as being embedded in the WLMs the polymer avoids unfavorable contacts with water while gaining some conformational freedom compared to a collapsed conformation in bulk water. The incorporation of P4VP can be favorable for the micelles too, since the polymer can partially shield the alkane tails from contacts with water and partially screen electrostatic repulsion between similarly charged surfactant head groups. Complexes of WLMs formed by the anionic surfactant potassium oleate with P4VP macromolecules have been characterized using several experimental techniques (dynamic and static light scattering, SANS, cryo-TEM, UV-spectroscopy and rheology) by the group of Prof. Philippova (Moscow State University, Russia).
Figure. 1: Simulation snapshot of a potassium oleate worm like micelle with an embedded P4VP polymer of 60 repeat units in 6 wt % KCl aqueous solution. P4VP is shown in blue, the surfactant tail in olive, and oxygens of the headgroup in red. Zoom-in pictures (top) show a local surfactant arrangement near the P4VP chain and water hydrogen bonded to nitrogen of P4VP in an atomistic representation (left) and surfactant surface representation (shown in green, right). Other water molecules and ions are not shown for clarity.
SANS contrast variation reveal that the micelles keep their locally cylindrical structure, whereas the macromolecules have an expanded coil-like conformation, in agreement with our molecular dynamic simulations. It has been found that presence of polymer affects significantly the rheology of the surfactant solution. At small concentration of P4VP the viscosity slightly decreases while the plateau modulus remains almost constant indicating little change in the network of entangled surfactant micelles. When the concentration of P4VP becomes higher than one polymer per elastically active micellar strand, a pronounced drop of viscosity by more than 4 orders of magnitude is observed indicating a full disruption of the network. In our jointly published paper we explain the drop of viscosity by polymer-induced shortening of WLMs accompanied by loop and/or branch formation. While further research in this area is needed to achieve a complete understanding of the system behavior, the obtained results demonstrate that a polymer, which has no specific favorable interactions with the WLM core, can be solubilized by WLMs and alter the thermodynamic and rheological properties of the surfactant network. Taking into account that such hybrid aggregates are quite promising building blocks for the construction of various self-assembled systems, our results will also offer guidance in designing new responsive materials employing WLMs and polymers.
We have also studied using atomistic molecular dynamics simulations the interaction of the more hydrophobic polymer polystyrene (PS) with a wormlike micelle (WLM) formed by the anionic surfactant potassium oleate in the presence of 6 wt % potassium chloride (KCl). Our initial results indicate that polystyrene enters WLM and is encapsulated inside the micelle in the surfactant tail area, as shown in Figure 2.
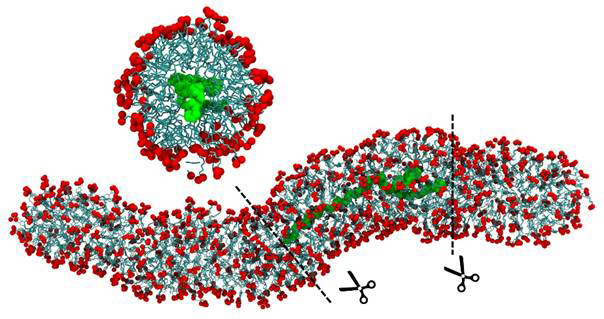 |
Figure. 2: Simulation snapshot of a potassium oleate worm like micelle with encapsulated inside polystyrene chain of 60 repeat units in 6 wt % KCl aqueous solution. PS is shown in green, the surfactant tail in cyan, and oxygens of the headgroup in red. Top image represents the perpendicular cross -sectional view of the section between the dashed lines of WLM with encapsulated polymer. Water and ions are not shown for clarity.
Working on this project has stimulated the PI to learn more about the current research in development in the area of surfactant self-assembly. The graduate student working on the project has learned both coarse-grained and especially atomistic molecular dynamics simulations in application to surfactant systems, which has expanded his knowledge of simulation techniques and surfactant system behavior. This project also has strengthened our collaboration with a leading experimental group and has allowed us to test stimulation models developed in the PI’s group. Overall, the project has contributed to the professional development of the PI and graduate students from the PI’s group in a new area of soft matter physics.