Reports: DNI1054190-DNI10: A Novel Integrated Design for the Mixed Ionic-Electronic Conducting (MIEC) Perovskites
Yu Zhong, Florida International University
In the current project, the CALculation of PHAse Diagram (CALPHAD) approach was adopted for the thermodynamic calculations. The CALPHAD approach was pioneered by Kaufman to model complex phase equilibria in multicomponent systems. Its theoretical basis is thermodynamic description of individual phases.
The thermodynamic database was developed/combined using Thermo-Calc. Thermo-Calc is a program developed by KTH Royal Institute of Technology in the 1970s. For over 40 years, Thermo-Calc has been at the forefront of scientific software and databases for calculations involving computational thermodynamics.
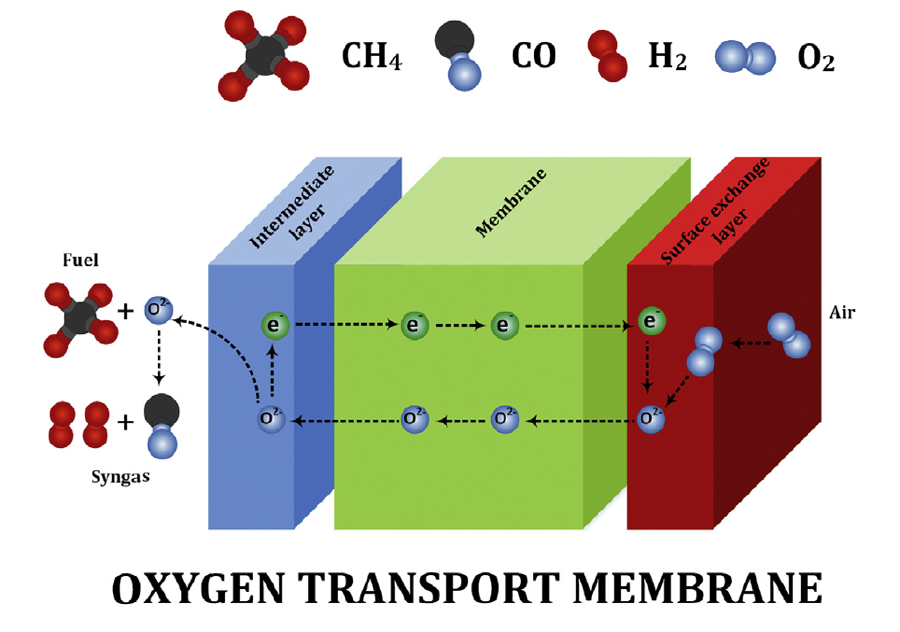
Figure 1. Schematic of oxygen transport membrane
Figure 1 shows a schematic of oxygen transport membrane. The system consists of three layers i.e. air electrode (surface exchange layer), oxygen transport membrane and fuel electrode (intermediate layer). Oxygen molecules from air dissociate into oxygen ions at the air electrode, migrate through the membrane, and recombine with the electron to form oxygen molecule on the fuel side. The driving force for the selective oxygen transport is the existence of oxygen partial pressure gradient. Air and fuel electrodes are used to enhance surface exchange kinetics and improve the oxygen flux performance of OTM system. Wide spread implementation of the aforesaid, however, remains critically limited by the current unfavorable economics, unproven reliability and lack of longevity. The thermodynamic understanding of the perovskite like (La,Sr)(Cr,Mn)O3 and fluorite like YSZ are thus very important.
1. Thermodynamic assessment of the chemical stability of (La0.8Sr0.2)0.98CrxFe1-xO3±δ under oxygen transport membrane fabrication and operation conditions
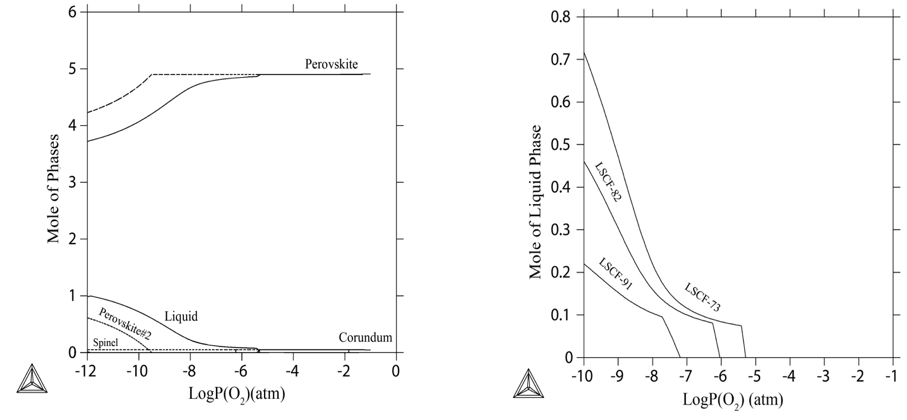
A comprehensive research has been conducted to evaluate and predict the chemical stability of (La0.8Sr0.2)0.98CrxFe1-xO3±δ (LSCrF, x=0.7, 0.5, and 0.3) under reducing and oxidizing atmospheres. The phase stability of the perovskite phase and behavior of liquid phase mainly depend on Fe content in the phase so that increase in Fe content in the perovskite phase can aggravate chemical stability and promote the formation of the liquid phase under reducing atmosphere. A threshold diagram (Figure 2) has been provided specially for the formation of the liquid phase with respect to changes in temperature, oxygen partial pressure, and composition. It can be used as a map for sintering process.
Figure 2. Threshold diagram for 0.2 mol of the liquid phase with the change in temperature and P(O2).
2. Role of chromium: Iron ratio and oxygen partial pressure on the processing and chemical stability of iron doped lanthanum strontium chromite based OTM
The focus of the research is to examine densification, microstructural changes, and chemical stability of (La0.8Sr0.2)0.95CrxFe1-xO3±δ (LSCrF, x=0.9, 0.8, and 0.7) through change in Cr:Fe ratio and oxygen partial pressure. FeOx and Fe1+xCr2-xO4 are characterized by X-ray diffraction as the secondary phases when LSCrF was exposed to 1400°C and 10-10 atm. Compared to other LSCrF compositions with higher Cr content, the stability of LSCrF has been aggravated as Fe content increases inside the perovskite phase. Indeed, LSCrF with x=0.9 (the highest Cr content) reveals the highest chemical stability under OTM conditions. In contrary, the densification of the final LSCrF samples have been improved for all three atmospheres (air, nitrogen, and Ar-3% H2- 3% H2O) as Fe content increases for initial LSCrF samples. The thermodynamic simulation results prove the liquid phase formation is being promoted as Fe content increases in the initial perovskite phase (Figure 3). In addition, the thermodynamic assessment also covers that the solidification behavior of the liquid phase will change by change in cooling rate so that two kinds of perovskites with slightly different compositions can solidify from the liquid phase under equilibrium cooling rate.
Figure 3. (a) Thermodynamic prediction of the phase stabilities of LSCrF73 with different oxygen partial pressure at 1400 °C (solid lines) and 1000 °C (dash lines); (b) Thermodynamic prediction of the liquid formation at 1400 °C with different oxygen partial pressure.
3. Thermodynamic Stability Maps for the La0.6Sr0.4Co0.2Fe0.8O3-CO2-O2 System
The thermodynamic predictions regarding the phase formation in LSCF-6428 system in wide range of temperature, P(CO2), P(O2), and LSCF composition have been conducted and compared with the previous investigations. According to the predictions, SrCO3, (Co,Fe)3O4 and Fe2O3 may precipitate from LSCF with the CO2 exposure to especially at low temperatures. The threshold diagram for the SrCO3 stability in the LSCF system is plotted in Figure 4 for the first time. During the simulations, the threshold was defined as SrCO3 is a stable phase, however, its concentration is zero. It is also seen in the threshold diagrams that by decreasing the temperature, as well as by increasing the P(CO2) and Sr concentration, the propensity to form SrCO3 increases. Overall, Thermal-cycle is considered as the main reason for SrCO3 formation, which may block the oxygen reduction reaction (ORR) sites.
Figure 4. SrCO3 stability threshold diagram for SrCO3 stability for various LSCF compositions in N2
4. SrZrO3 Formation at the Interlayer/Electrolyte Interface During (La1-xSrx)1-δCo1-yFeyO3 Cathode Sintering
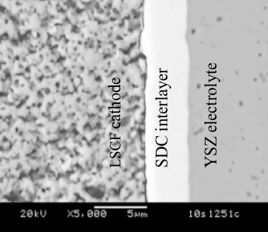
Formation of SrZrO3 at the SDC/YSZ interface (Sm doped ceria, SDC; Y stabilized zirconia, YSZ) during (La1-xSrx)1-δCo1-yFeyO3 (LSCF) cathode sintering has been investigated. It is well known that LSCF reacts with YSZ to form SrZrO3 when they are in physical contact with each other at high temperatures; However, even with the SDC interlayer physically separating the LSCF cathode from the YSZ electrolyte, SrZrO3 is formed by Sr migration through the SDC interlayer to the YSZ electrolyte as shown in Figure 5. Figure 6 demonstrates specifically the partial pressure of Sr-containing gas species for LSCF-6428 as a function of P(H2O) at 1200C and P(O2)=0.21atm. It is found that Sr(OH)2 has the highest partial pressure among all the Sr-containing species. In addition, by changing P(H2O) from 1E-5 to 0.1 atm, the partial pressure of Sr(OH)2 increases more than two orders of magnitude, which indicates that water vapor in air greatly facilitates the evaporation of Sr from LSCF. Overall, it has been suggested that the SrZrO3 formation is due to the Sr-containing gas species diffused through the pores of the SDC layer and reacted with the YSZ electrolyte.
Figure 5. Cross-sections of a dense SDC interlayer sintered at 1200C for 2 hours
Figure 6. Equilibrium partial pressure of Sr containing gas species as a function of (a) P(H2O) at 1200°C for LSCF-6428