Reports: DNI355281-DNI3: Metal-Organic Framework Supported Pincer Complexes: Investigation of the Effects of Site Isolation and Secondary Environment
Casey R. Wade, Brandeis University
Overview
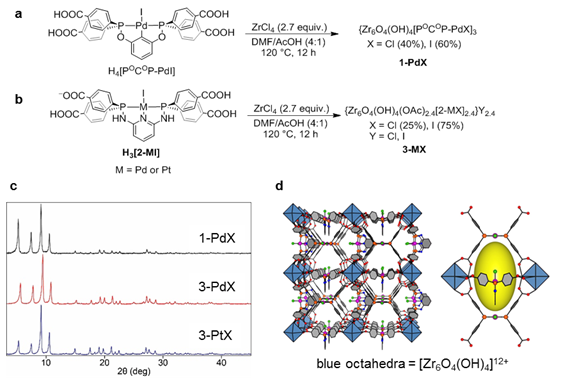
Our laboratory has been investigating the synthesis and reactivity of metal-organic frameworks (MOFs) assembled from linkers based on late transition metal diphosphine pincer complexes. The specific aims of this project are to” 1) elucidate design principles for the assembly of MOFs from organometallic pincer complexes; 2) investigate the effects of immobilization and site isolation on the reactivity and catalytic activity of organometallic species; 3) exploit site isolation and immobilization effects for the targeted design of improved catalytic materials. In year 1, we developed the synthesis of a Zr MOF (1-PdX, Figure 1a) constructed from POCOP-Pd metallolinkers and discovered that immobilization dramatically improves catalytic transfer hydrogenation activity compared to an analogous homogeneous complex. More recently, we have been investigating the reactivity and catalytic activity of Zr MOFs assembled from linkers based on PNNNP-Pd and PNNNP-Pt pincer complexes. A postsynthetic metal exchange strategy has also been developed and promises to be a versatile alternative to de novo synthesis of pincer MOFs.
Figure 1. a) Synthesis of 1-PdX. b) Synthesis of 3-PdX and 3-PtX. c) Powder X-ray diffraction patterns showing the isostructural relationship among 1-PdX, 3-PdX, and 3-PtX. d) Framework structure of 3-PdX and 3-PtX.
Progress
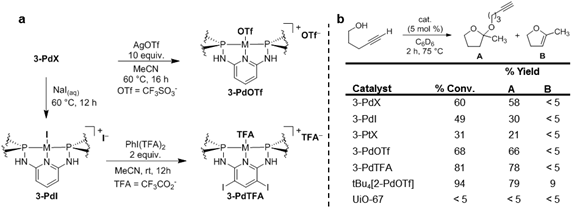
Solvothermal reaction of the PNNNP complexes H3(2-PdI) and H3(2-PtI) with ZrCl4 generates MOFs 3-PdX and 3-PtX, respectively (Figure 1b). Powder X-ray diffraction (PXRD) analysis indicates that these MOFs are isostructural to 1-PdX, and should have empirical formulas of {Zr6O4(OH)4[2-MX]3}Y3 based on the framework structure (Figure 1c,d). However, elemental analysis results combined with spectroscopic data suggest the presence of missing linker defects in 3-PdX and 3-PtX, and the empirical formulas are best given as {Zr6O4(OH)4(OAc)2.4[2-MX]2.4}Y2.4. A series of postsynthetic ligand exchange reactions have been carried out with 3–PdX, resulting in the generation of 3–PdI, 3–PdOTf, and 3–PdTFA (Figure 2a). The catalytic activities of these MOFs were compared for the intramolecular hydroalkoxylation of the terminal alkyne 4-pentyn-1-ol (Figure 2b). The results demonstrate that halide exchange for more weakly coordinating anions via halide abstraction with AgOTf or oxidative ligand exchange with PhI(TFA)2 results in increased catalytic activity. 3–PdTFA proved to be the most effective catalyst in the MOF series despite evidence for ligand-based iodination during the oxidative halide exchange. No substrate conversion was observed in the presence of UiO-67, indicating that the Zr6O4(OH)4(COO)12 clusters are not likely to be responsible for catalysis. A homogeneous analogue, tBu4[2-PdOTf], exhibits superior activity for the cyclization reaction, revealing that immobilization of the Pd pincer complexes tempers their Lewis acid catalytic activity.
Figure 2. a) Postsynthetic ligand exchange reactions of 3-PdX. b) Results of screening for the Lewis acid-catalyzed hydroalkoxylation of 4-pentyn-1-ol.
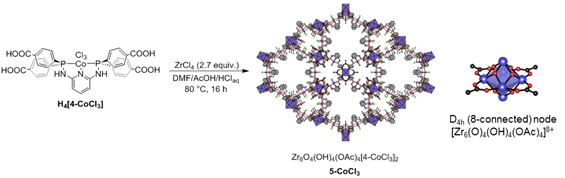
Solvothermal reaction of the PNNNP-Co pincer complex H4(4-CoCl3) and ZrCl4 results in formation of 5-CoCl3 as an orange microcrystalline powder (Figure 3). A structure solution for 5-CoCl3 has been obtained from PXRD data. Despite similar PNNNP pincer ligand scaffolds, 5-CoCl3 adopts a hexagonal framework structure rather than the cubic phase observed for 3-PdX and 3-PtX. The structural model of 5-CoCl3 shows 8-connected (D4h) Zr6 clusters joined by [4-CoCl3]4- linkers. The remaining four edges of the Zr6 octahedra are occupied by bridging acetate ligands. The structure contains two types of channels that are ~ 5 Å and ~10 Å in diameter, with the former providing access to the Co pincer sites.
Figure 3. Synthesis and framework structure of 5-CoCl3.
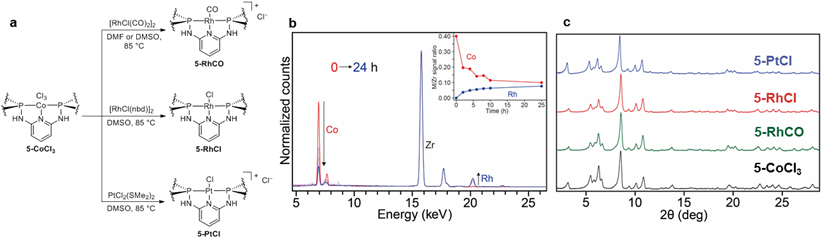
Demetallation processes and transmetallation reactions are not common for pincer complexes owing to the kinetic stability afforded by ligand chelation. However, in the course of our investigations into the reactivity of 5-CoCl3, we discovered that the PNNNP-CoCl3 linkers undergo facile transmetallation with softer late transition metals such as Rh(I) and Pt(II) (Figure 4a). A suspension of 5-CoCl3 in N,N-dimethylformamide (DMF) was treated with [RhCl(CO)2]2 at 85 °C, and samples of the MOF were periodically analyzed by X-ray fluorescence (XRF) spectroscopy. Over the course of 24 h, the XRF spectra show a significant decrease in the Co Kα signal at 6.9 keV concomitant with the appearance of a peak at 20.2 keV corresponding to Kα emission from Rh (Figure 4b). Subsequent PXRD analysis of the product (5-RhCO) shows that the framework crystallinity and structure are retained after the metal exchange (Figure 4b). Inductively coupled plasma atomic emission spectrometry (ICP-AES) data indicate a Zr:Co:Rh molar ratio of 3.5:0.04:1, confirming that the Co species have been replaced by Rh. NMR and IR spectroscopic studies have further confirmed that RhCO fragments are supported by chelation of the diphosphine pincer groups.
Postsynthetic metal exchange has also been employed for the synthesis of 5-RhCl and 5-PtCl. The direct synthesis of Zr MOFs containing PNNNP-Rh linkers has been precluded by the oxygen and acid sensitivity of the Rh complexes, which leads to incompatibility with ester deprotection reactions and solvothermal MOF assembly conditions. Preliminary catalytic screening shows that 5-RhCl is a competent catalyst for olefin hydrogenation under mild conditions (25 °C, 1 atm H2). Solvothermal reaction of H3(2-PtI) and ZrCl4 has been observed to generate the cubic MOF structure 3-PtX, but reaction conditions for direct synthesis of the hexagonal MOF structure 5-PtCl have not been identified. Thus postsynthetic metal exchange currently provides an exclusive means of accessing 5-RhCO, 5-RhCl, and 5-PtCl and represents a versatile approach to obtain pincer MOFs that are not accessible by direct solvothermal syntheses.
Figure 4. a) Postsynthetic metal exchange reactions of 5-CoCl3. b) Overlay of XRF spectra spectra measured during the Co/Rh metal exchange of 5-CoCl3 to form 5-RhCO. c) PXRD patterns of 5-CoCl3, 5-RhCO, 5-RhCl, and 5-PtCl.
Impact of Grant on PI’s Research Program
Over the past year, the funding provided by the ACS-PRF for this project has provided direct support for one postdoctoral research associate as well as supported research training for one graduate (PhD) student and one undergraduate researcher. So far, the project has resulted in one high profile publication, and three manuscripts are in preparation. Preliminary results obtained from work sponsored by this award have been included in proposals submitted to the National Science Foundation.