Reports: ND555377-ND5: Molecular Understanding of Clay Swelling in Enhanced Oil/Gas Recovery
Yongsheng Leng, PhD, George Washington University
Shale gas production with water-based hydraulic fracturing may invoke high water consumption and formation damage. Carbon dioxide (CO2) is a promising candidate for reservoir fracturing and enhanced oil-gas recovery (EOGR), plus benefit from long-term CO2 sequestration. Swelling clays are important components of shale minerals. Understanding the interaction between water–carbon dioxide–methane (CH4) ternary mixture and swelling clays is important for CO2-EOGR in shale gas reservoirs.
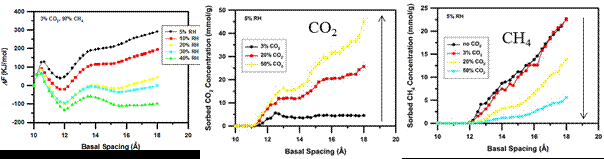
In the second year research, we employed the grand-canonical Monte Carlo (GCMC) and molecular dynamics (MD) simulations to investigate a H2O–CO2–CH4 ternary mixture in Na-montmorillonite clay interlayer under geological conditions (T = 323 K, P = 90 bar) with relative humidity (RH) in the ranges of 5% – 40%. In one case, we consider the very high mole fraction of methane in the bulk (3% CO2 and 97% CH4) and studied the effects of RH on the intercalation of different species. The stable clay interlayer distances at different RH values are determined based on the calculated free energy curves of the clay-fluid complex system. We find that the H2O–CO2–CH4–Na complex largely stays in the monolayer (1W) hydration with a basal spacing around 12 Å, see Figure 1a.
(a)(b)(c)
Figure 1. (a) Variations of free energy of the ternary system as a function of basal spacing at different RH; (b) and (c) show at 5% RH the competition of sobered CO2 and CH4 amount in clay interlayer versus CO2 mole fraction in the bulk.
Our simulation results show that the intercalation of CH4 is strongly outcompeted by CO2 upon the hydration of interlayer sodium ions by water molecules. However, low RHs such as RH = 5% do provide opportunities for the co-sorption of CH4 with CO2. The competitive sorption between CO2 and CH4 in clay interlayers depends on the CO2/CH4 ratio in the ternary mixture. As shown in Figure 1b and 1c, as the CO2/CH4 ratio increases, the sorption of CO2 increases while CH4 sorption decreases. Our major findings concerning this competition of sorption are: (1) intercalation begins in a spacing sequence of H2O (10.5 Å) → CO2 (11.5 Å) → CH4 (12.5 Å); (2) affinity to clay interlayer follows H2O > CO2 > CH4; and (3) H2O has the highest mole fraction in clay interlayer, followed by CO2 and CH4. MD simulations show that CO2 and CH4 molecules are partially hydrated, especially at low RHs, while sodium ions are fully hydrated due to its large hydration energy with water molecules. Further, some CO2 molecules can migrate into the first hydration shell of sodium ions at low RHs (< 20%). These findings provide better understanding of the behavior of H2O–CO2–CH4 ternary mixture in clay interlayers and demonstrate the potential implementation of CO2-based EOGR in shale gas reservoirs.
We also investigated the effect of clay interlayer charge, including the charge amount and charge location (either in octahedral layer or in both octahedral and tetrahedral layers), on the swelling behavior of montmorillonite in wet CO2. Two clay models with different layer charges are used in the simulation. The first clay model is an Arizona-type montmorillonite with the chemical formula Na1.0[Si8.0](Al3.0Mg1.0)O20(OH)4, which only has isomorphic substitutions in the octahedral site (octahedral charge deficit) and was used in previous simulation studies. The second clay model is a Wyoming-type montmorillonite with the chemical formula Na0.75[Si7.75Al0.25](Al3.5Mg0.5)O20(OH)4, which has isomorphic substitutions in both octahedral and tetrahedral sites The two clay models have an interlayer charge per unit cell –1.0 e and –0.75 e, respectively.
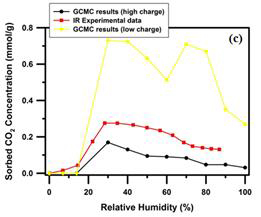
We consider typical geological condition T/P = 323 K/90 bar, under which experimental data are available for comparison. We find that both high relative humidity and high layer charge facilitate water molecules entering clay interlayers and decrease the CO2 intercalation. In Figure 2a, we plot the equilibrium basal spacings versus different RH values for both high-and low-charge clay models. In-situ X-ray diffraction (XRD) experimental results for Na-SWy-2 clay sample are also shown in the figure for comparison. For the two clay models studied here, the 1W and 2W equilibrium basal spacings are not quite dependent on layer charge due to possible counteracting effect of the charge amount and charge location. The sorbed H2O and CO2 concentrations for the two clay models are shown in Figure 2b and 2c. In-situ IR spectroscopy experimental results are also shown in the figures for comparison. The high-charge clay contains more water than the low-charge clay in both 1W and 2W states. Water concentration in the high-charge clay also has a large shift toward low RH values (Figure 2b). CO2 concentration in the high-charge clay is dramatically decreased, which is clearly shown in Figure 2c, in which the high-charge CO2 concentration curve is even below the IR experimental curve. Since the CO2 concentration curve of the low-charge clay is well above the experimental data (Figure 2c), this scenario suggest that, perhaps, the clay samples used in the in-situ IR spectroscopy experiment is a combination of low-charge and high-charge clays with different stoichiometry. The fact that high-charge clays containing more water and less CO2 as the RH increases is associated with the increased number of interlayer cations and the strong hydration energy of sodium ions with water molecules. This enhanced hydration process reduces the chance of intercalations of CO2 molecules.
Figure 2. Variations of (a) equilibrium basal spacing, (b) sorbed H2O concentration and (c) sorbed CO2 concentration as a function of the relative humidity for the high- and low-charge Na-montmorillonite clay at T/P = 323 K/90 bar. The XRD and IR experimental data obtained from PNNL Loring research team are also shown in the figure for comparisons.