Reports: DNI156878-DNI1: A New Class of Reagents for Heterocycle Coupling Reactions
Andrew McNally, PhD, Colorado State University
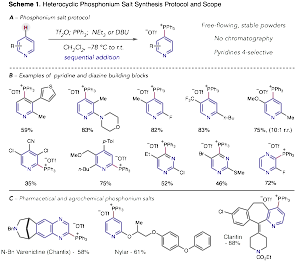 Formation of heterocyclic phosphonium salts. The first key aspect of the project was to develop a general method to transform pyridines and diazines into heterocyclic phosphonium salts. This goal has been achieved in full and Scheme 1 shows that a diverse set of these heterocycles can undergo salt formation via the describes protocol. In the vast majority of cases, the reaction is 100% regioselective for the 4-position of pyridines even in systems with steric encumbrance on the adjacent atoms. The salts are isolated via a simple precipitation process without any requirement for chromatography. As well as building block heterocycles, we have also shown that this process can be applied to complex pharmaceuticals and agrochemicals. This part of the project in conjunction with a C-O coupling reaction (vide infra), was published, J. Am. Chem. Soc. 2016, 138, 13806 before the grant period and effectively serves as an underpinning study for the remaining chemistry in the proposal.
Formation of heterocyclic phosphonium salts. The first key aspect of the project was to develop a general method to transform pyridines and diazines into heterocyclic phosphonium salts. This goal has been achieved in full and Scheme 1 shows that a diverse set of these heterocycles can undergo salt formation via the describes protocol. In the vast majority of cases, the reaction is 100% regioselective for the 4-position of pyridines even in systems with steric encumbrance on the adjacent atoms. The salts are isolated via a simple precipitation process without any requirement for chromatography. As well as building block heterocycles, we have also shown that this process can be applied to complex pharmaceuticals and agrochemicals. This part of the project in conjunction with a C-O coupling reaction (vide infra), was published, J. Am. Chem. Soc. 2016, 138, 13806 before the grant period and effectively serves as an underpinning study for the remaining chemistry in the proposal.
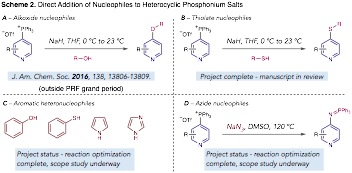 Reaction of Heterocyclic Phosphonium Salts with Nucleophiles. Our initial publication (outside of the grant period) involved adding alkoxide nucleophiles to heterocyclic phosphonium salts as a new strategy to make heteroaryl ethers (Scheme 2A). Building on this progress during the grant period, we have discovered three different classes of additional nucleophiles that can also engage with these salts and result in valuable pyridine and diazine derivatives. Thiolate nucleophiles are competent nucleophiles and results in valuable heteroaryl thioethers; a complete study of this reaction was recently completed and a manuscript was submitted in response to a special edition of Tetrahedron (Scheme 2B). Aromatic heteronucleophiles, such as phenols, thiophenols, pyrroles and imidazoles are a distinct class of nucleophile that also undergo productive coupling (Scheme 2C). In this project, we are currently exploring the scope of the reaction and anticipate the project to be complete in 6 weeks. Finally, azides can react to form C-N bonds and with a useful iminophosphorane functional group in the product (Scheme 2D). This project is also at the scope stage and close to completion.
Reaction of Heterocyclic Phosphonium Salts with Nucleophiles. Our initial publication (outside of the grant period) involved adding alkoxide nucleophiles to heterocyclic phosphonium salts as a new strategy to make heteroaryl ethers (Scheme 2A). Building on this progress during the grant period, we have discovered three different classes of additional nucleophiles that can also engage with these salts and result in valuable pyridine and diazine derivatives. Thiolate nucleophiles are competent nucleophiles and results in valuable heteroaryl thioethers; a complete study of this reaction was recently completed and a manuscript was submitted in response to a special edition of Tetrahedron (Scheme 2B). Aromatic heteronucleophiles, such as phenols, thiophenols, pyrroles and imidazoles are a distinct class of nucleophile that also undergo productive coupling (Scheme 2C). In this project, we are currently exploring the scope of the reaction and anticipate the project to be complete in 6 weeks. Finally, azides can react to form C-N bonds and with a useful iminophosphorane functional group in the product (Scheme 2D). This project is also at the scope stage and close to completion.
 Metal-Catalyzed Cross-Coupling Reactions. We aimed to show that the phosphonium ion could serve as an equivalent to a halide and enable metal catalyzed cross-coupling reaction with aryl and alkyl coupling partners. In our first endeavor, we have successfully developed a Ni-catalyzed cross-coupling reaction of heterocyclic phosphonium salts with aryl boronic acids to form heterobiaryl products (Scheme 3). A broad range of
Metal-Catalyzed Cross-Coupling Reactions. We aimed to show that the phosphonium ion could serve as an equivalent to a halide and enable metal catalyzed cross-coupling reaction with aryl and alkyl coupling partners. In our first endeavor, we have successfully developed a Ni-catalyzed cross-coupling reaction of heterocyclic phosphonium salts with aryl boronic acids to form heterobiaryl products (Scheme 3). A broad range of 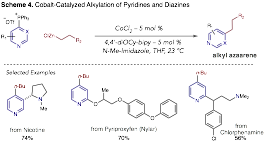 coupling partners are viable in this process and the study includes examples that would be outside the scope of traditional cross-coupling approaches. This project is complete and was recently published (Angewandte Chem. Int. Ed. 2017, 56, 9833) and rated as a Very Important Paper.
coupling partners are viable in this process and the study includes examples that would be outside the scope of traditional cross-coupling approaches. This project is complete and was recently published (Angewandte Chem. Int. Ed. 2017, 56, 9833) and rated as a Very Important Paper.
We have subsequently discovered a method to alkylated pyridines and diazines via heterocyclic phosphonium salts using alkylzinc organometallics as coupling partners (Scheme 4). The Ni-catalyzed conditions developed above were ineffective for this process and resulted in a mixture of heterocycle and phenyl transfer products. Surprisingly, testing cobalt salts as catalysts in conjunction with bipyridine type ligands results in an efficient process. Modifying the bipyridine ligand scaffold to incorporate cyclohexyloxy substituents was critical for high yields in this coupling reaction. At present, optimization studies have been completed and we are currently exploring the generality of this reaction by testing a variety of pyridine and diazine-derived phosphonium salts and organozincs coupling partners.
Photoredox Coupling Reactions. We have recently obtained a breakthrough result that demonstrates that heterocyclic phosphonium salts can be coupled other electron-deficient heterocycles using photoredox catalysis (Scheme 5). Our attempts to couple phosphonium salts with alkenes or electron-rich aromatics were  unsuccessful. However, a specific combination of photoredox catalysts, organic base and a boron reagent as an additive allows coupling of the phosphonium salt to another pyridine via its 2-position C-H bond. We believe that Ru(bpy)3Cl2 is excited by visible light and in turn sensitizes pyrene. The exited state pyrene then accepts and electron from the organic base and reduces the C-P bond to form a heteroaryl radical. Simultaneously, activation of 4-cyanopyridine with B2Pin2, via a Lewis acid-Lewis base pair adduct, enables coupling at the 2-position of the scaffold. We believe this is the first reaction of its type that can selectively couple two pyridines using photoredox catalysts. Currently, we are optimizing the reaction and will subsequently investigate the scope of the process.
unsuccessful. However, a specific combination of photoredox catalysts, organic base and a boron reagent as an additive allows coupling of the phosphonium salt to another pyridine via its 2-position C-H bond. We believe that Ru(bpy)3Cl2 is excited by visible light and in turn sensitizes pyrene. The exited state pyrene then accepts and electron from the organic base and reduces the C-P bond to form a heteroaryl radical. Simultaneously, activation of 4-cyanopyridine with B2Pin2, via a Lewis acid-Lewis base pair adduct, enables coupling at the 2-position of the scaffold. We believe this is the first reaction of its type that can selectively couple two pyridines using photoredox catalysts. Currently, we are optimizing the reaction and will subsequently investigate the scope of the process.
Impact
Impact on the PI's career. The PRF grant has been instrumental in launching the research program in my laboratory. As described in this report, a number of projects have emerged during the funding period thus far including two completed projects. It has enabled me to deliver several lectures (ACS San Francisco & Philadelphia, Heterocycles GRC, Canadian Society of Chemistry 100th Anniversary Conference) to disseminate these results to the chemical community with several invited talks planned for next spring. My laboratory has been able to build up a distinct program in heterocyclic chemistry and enabled my group to submit grants to other agencies in related work.
Impact on Students. One postdoctoral researcher, Xuan Zhang and has been supported on this grant. Dr. Zhang has gained one publications and will complete other project in this portfolio in the next funding year. He is applying for industrial position in the US next spring. Michael Hilton, a graduate student was funded on the grant for a 3 month period and is engaged in the nucleophile addition projects. I was also able to support two outstanding undergraduates; Brianna Jet completed the thiolation project that was recently submitted for publication and Margaret Mohnike is working with azide nucleophile as partners. Both students are now fully integrated into my research group and have gained vital experience before they apply for graduate school.











