Reports: ND656680-ND6: Vibrational pre-reactivity: exploiting complexes to probe reactive resonances
Craig Murray, PhD, University of California, Irvine
This project aims to explore the dynamics associated with reactive resonances in bimolecular reactions. Our approach exploits the van der Waals character of the resonance states, by first preparing atom-molecule complexes and then using tunable infrared radiation to optically prepare the resonance states. The resonances can decay by predissociation (returning to reactants) or prereaction (forming products), and the dynamics of these processes will be explored by using velocity-map-ion imaging to measure the correlated product state distributions.
Progress towards these goals has been a little slower than hoped, largely due to a succession of technical problems that extended the time required to complete a project that was underway at the start of the award. A scroll pump backing a turbomolecular pump on the imaging apparatus failed, a series of new solenoid pulsed vales were found to be defective and had to be returned for replacement, and an initially misdiagnosed problem with the electronics in the photolysis laser ultimately required it be returned to the manufacturer for servicing. These issues are now resolved. The imaging spectrometer has been modified to allow photolysis of atom or radical precursor species in the throat of the supersonic expansion, prior to the skimmer and a new gas and liquid sample handling manifold has been constructed to allow safe handling of precursor chemicals. The tunable infrared optical parametric oscillator (OPO) and its injection-seeded Nd:YAG pump laser had been long-neglected and significant amount of work has been required to achieve performance close to specification. The non-resonant oscillator of the OPO is now performing well and working reliably, producing the expected pulse energies with a bandwidth of 3 cm–1 in the mid-IR. The single-mode oscillator in this OPO model is notoriously fussy, and while it has proven challenging to achieve reliable mode-locking, the narrow band output is desirable rather than essential for the project. We have also constructed a photoacoustic cell, and associated electronics, to allow measurement of spectra of water vapor in the mid-IR for wavelength calibration. While we have not yet observed complexes, preliminary HCl REMPI spectra confirm that good cooling (~12 K) is being achieved in the supersonic expansion and that SOCl2 is a viable precursor for generation of Cl atoms. All the necessary pieces are now in place and we anticipate significant progress in the coming months.
At the start of the award period, the graduate student funded by this award was in the early stages of a project exploring the photodissociation dynamics of acetaldehyde cation. This project was a natural and straightforward successor to a recently completed set of experiments exploring the near ultraviolet (UV) photochemistry of neutral acetaldehyde and acetone, as it involved a simple switching of the time-order of the photolysis and ionization laser pulses. Where we had been using single photon vacuum ultraviolet (VUV) ionization at 118 nm to detect photolysis products, we instead used it to prepare cold CH3CHO+ cations that subsequently could be dissociated with the UV pulse. As the apparatus was set up to use and working with the static gas VUV mixing cell, we made the decision to wrap up these experiments before making any fundamental changes to the spectrometer, while in parallel working on the IR OPO and taking other preparatory steps.
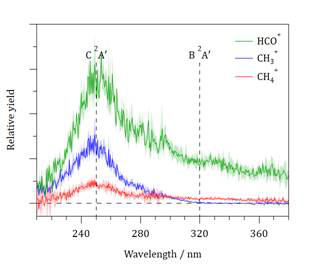
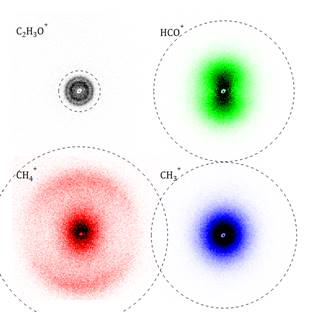
Ionization of CH3CHO at 118 nm produces vibrationally cold cations, with most of the population found in the zero-point level. The VUV photon alone provides insufficient energy to cause fragmentation and the mass spectrum contains a single feature at m/z = 44. Introduction of the UV laser pulse induces fragmentation; C2H3O+, HCO+, CH4+ and CH3+ are the major products. Figure 1 shows photofragment yield spectra obtained by scanning the photolysis laser wavelength over the range 210–380 nm. CH3+ ions are observed only at λ ≤ 320 nm, which corresponds to the expected wavelength required to reach the B2A′ state of CH3CHO+. HCO+ and CH4+ are observed across the wavelength range. The maximum yields for all ions occur at ~250 nm. Ion images of the four major fragment ions have been measured between 208–316 nm; images obtained at 236 nm are shown in Figure 2. At all photolysis wavelengths, CH3+ ions are formed with a statistical translational energy, ET, distribution and isotropic angular distribution, while the major HCO+ products have angular distributions that show increasing anisotropy for the faster ions. The C2H3O+ ET distribution comprises a single feature at longer wavelengths, but three components at shorter wavelengths, with average energies separations being consistent with opening of isomerization channels. The CH4+ images are most intriguing, comprising exclusively an isotropic and statistical component at longer wavelengths, while a fast moving and highly parallel component is evident at λ < 300 nm.
Figure 1 Photofragment yield spectra obtained following UV photolysis of CH3CHO+. The dashed vertical lines indicate the estimated origins of the B2A′ and C2A′ states.
Figure 2 Ion images of C2H3O+, HCO+, CH4+, and CH3+ produced by photolysis of CH3CHO+ at 236 nm. Dashed circles indicate the maximum possible speed for each fragment ion.