Reports: UR453159-UR4: Impact of Solvent-Solute Interactions on Photochemistry of p-Aminobenzoic Acid Derivatives
Sarah J. Schmidtke Sobeck, PhD, College of Wooster
Our research is focused upon the impact of environment and structure on the physico-chemical properties of para-aminobenzoic acid (PABA) derivatives. A general scheme for the potential photo-induced intramolecular charge transfer (ICT) is shown in Figure 1. This year a primary focus has been upon completing our studies focused upon the impact of modifying the electron acceptor moiety. We optimized the synthesis of the thionoester and halogenated esters, and completed analysis of their spectroscopic properties. Secondly we initiated studies looking at the impact of modifying the positioning of the electron donor and acceptor moieties (ortho-, meta-, para-) upon the photochemistry in the parent aminobenzoic acid compounds.
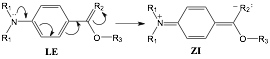
Figure 1. Potential ICT reaction from the locally excited (LE) to zwitterionic (ZI) states for PABA derivatives.
Results
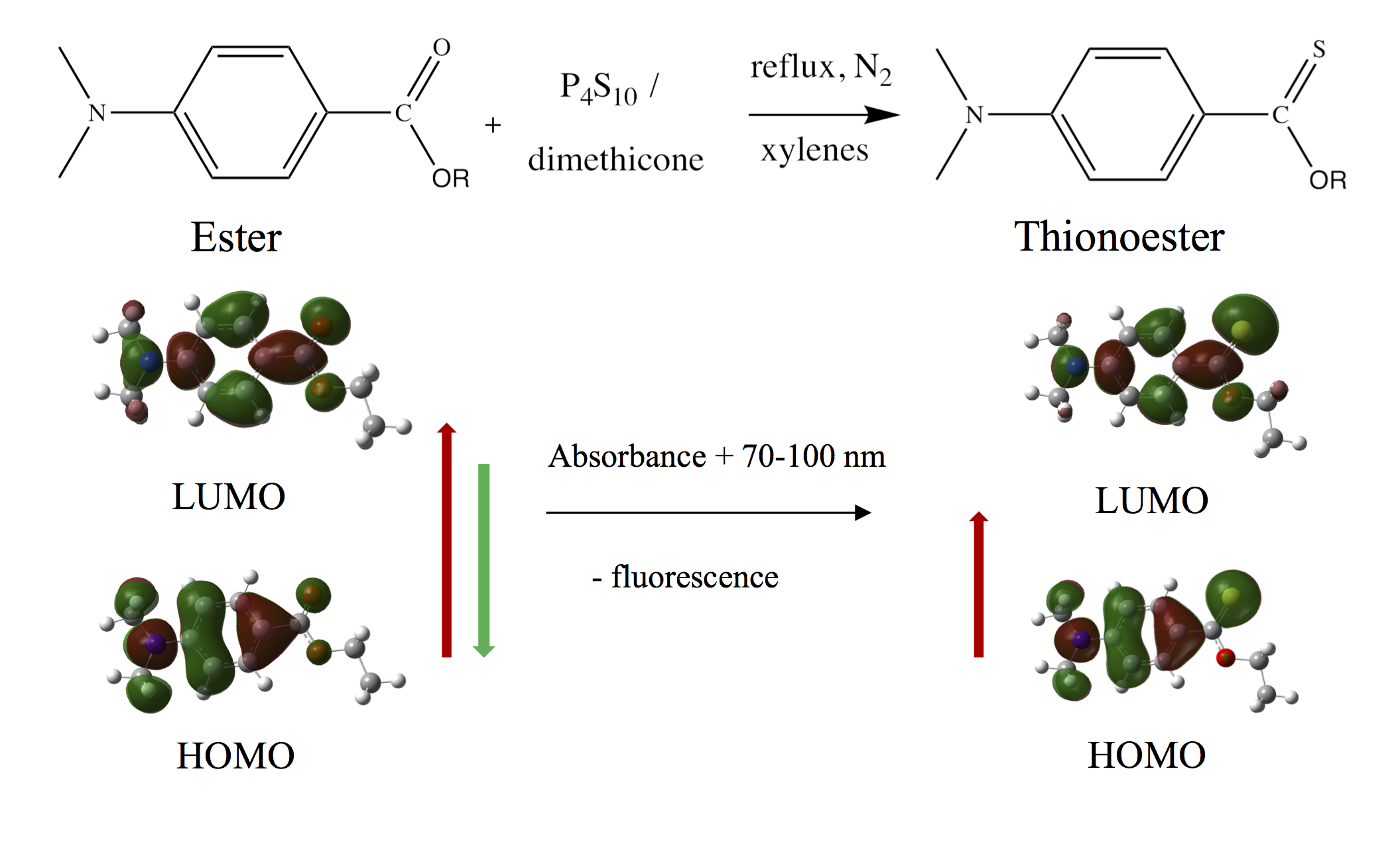
During the 2016-17 academic year, two students continued their work on the PABA system. Haley Rossiter finalized her analysis of the compounds she had previously synthesized, describe in past narratives. Our paper summarizes the synthesis and photophysical properties of the series of aminobenzoates, and includes 3 undergraduate student co-authors. This paper is currently in press and the graphical abstract is in Figure 2.
Figure 2. Graphical abstract summarizing our synthetic and characterization work on the aminobenzoate derivatives.
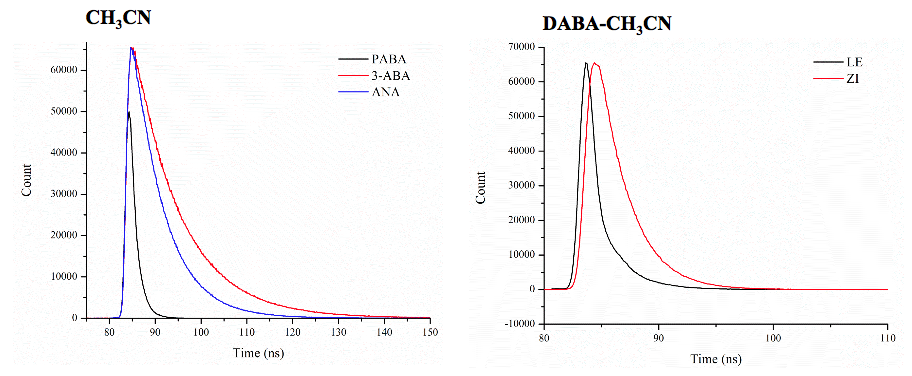
Secondly, Haley initiated a study assessing the photochemistry of a series of aminobenzoic acid derivatives varying the positioning of the amine donor and acid acceptor moieties. She evaluated the impact of solvent on the absorbance and emission of the compounds, as well as carrying out time-resolved fluorescence measurements to evaluate the lifetime of the systems (Figure 3). The latter measurement was carried out using equipment at Ohio Northern University. Haley and I traveled to the facilities to carry out these measurements during the 2017-18 winter recess. The results indicate the primary amines do not exhibit charge transfer, but that tertiary amines do exhibit charge transfer. The ortho-substituted compounds have variations in its properties that are attributed to the strong intramolecular interactions between the amine and carboxylic acid moieties. Computational modeling was used to support this assignment. Further work is planned to evaluate the properties of this series in a greater range of solvents.
Figure 3. Time-resolved emission profiles for benzoic acid derivatives. The spectrum on the left shows variation in emission decays for the primary amines with substitutes positioned para- (PABA, meta- (3-ABA), and ortho- (ANA) relative to the carboxylic acid. The spectrum on the right shows the unique emission decay curves for fluorescence from the locally excited (LE) state and charge transfer state (ZI) of para-dimethylaminobenzoic acid (DABA). Together this illustrates the impact of both substituent position and presence of ICT on fluorescence lifetimes.
My sophomore research assistant, Gabriela Jocas, continued the UV-degradation studies comparing the solvent and ambient environment dependence of the degradation of PABA and DABA (dimethyl-aminobenzoic acid). This is work she initiated in the summer following her first year, supported by this research grant. In this academic year, she worked on repeating trials and beginning evaluation of the impact of anoxic conditions on the decay rates. This is the initial work in evaluating potential mechanisms of degradation, particularly evaluating the role of reactive oxygen species in the process.
Student Impact
During this last academic year five of my research students, including Haley and Gabriela, presented at the National American Chemical Society Meeting in San Francisco. Haley was also an author on my oral presentation at this meeting, as well as my contributions to the Photochemistry Gordon Research Conference and Inter-American Photochemical Society Winter Meeting. Her work is also being incorporated into a talk I am giving this fall at Bowling Green State University.
Ongoing Plans
During the present academic year, Gabriela Jocas is studying abroad in Scotland, but she may return to the project in the spring semester upon her return to campus. I have hired a sophomore research student to continue the work that Haley Rossiter evaluating the solvatochromic behavior of the aminobenzoic acids varying by substituent positioning. Additionally, I am advising two Senior Thesis students and one Junior Independent Study student. The Junior Independent Study student is developing his Independent Study project this semester and will initiate labwork in the spring. His work will continue the studies of Briana Marlatt (described in the 2016 narrative) assessing the solvent-dependence of the photodegradation of PABA versus an ester derivative that undergoes ICT. My two Senior Independent Study students are analyzing the photochemistry of dyes and pigments focusing upon organic (anthraquinone) dyes and pigments using in cultural heritage and in foods. They are developing HPLC methods to separate degradation products and gain more insight into chemical mechanisms of photodegradation. Similar methods may be applied to the benzoic acid studies in the future.
This coming summer I intend to hire several student researchers to continue on this work. This is following a summer without researchers due to construction delays impacting facilities in my laboratory. The chemistry building is interfacing to a new life science center on campus which has resulted in periodic disruptions to our utilities (water/power/air handling) and minimal activities in chemistry over the past summer. The new building is set to open in the fall of 2018, so fewer disruptions are anticipated as the project comes to a close this summer. I am very grateful for the extension to this grant to accommodate for the disruptions.
I will be working on drafting a paper in the work that Haley initiated this past year on the impact of donor/acceptor group positioning on the observed photochemistry. I also plan to present at the Winter I-APS meeting again this January, presenting on our ongoing studies of the benzoic acid systems.
The PI and her group continue to appreciate the generous support from the ACS PRF grant. This support makes the full-time (immersive) summer research experience possible, trips to instrumentation to enrich our studies, and conference participation. All of which are contribute greatly to the scientific development of our students.