Reports: ND753960-ND7: Well-Defined Polyrings via Diyne-Functionalized Cyclic Monomers
Chong Cheng, PhD, State University of New York at Buffalo
The goal of this project is to establish a method for the synthesis of well-defined polyrings with a sizable ring per repeat unit, which are model polymers ideal for the study of the effect of side ring structures on polymer properties. The spacers between backbone and side rings of polyrings should be either absent or very short, in order to eliminate or reduce the effect from linear portions of side groups. We are particularly interested in polyrings with oligo(ethylene glycol) (OEG)-based rings because they are expected to possess special guest-host behavior and environmental responsivity. As revealed in our previous studies, the separation of OEG-based cyclic monomers from reaction mixtures is very difficult when ring-closure reactions are used (the efficiency of such reactions is limited, but the targeted product and side products have very similar Rf values in chromatography). In the past grant year (9/1/2016 – 8/31/2017), the separation difficulties could not be overcome although continuous efforts were made. Therefore, the research focus was shifted to the synthetic route to polyrings via crown ether-based monomers without involving ring-closure steps.
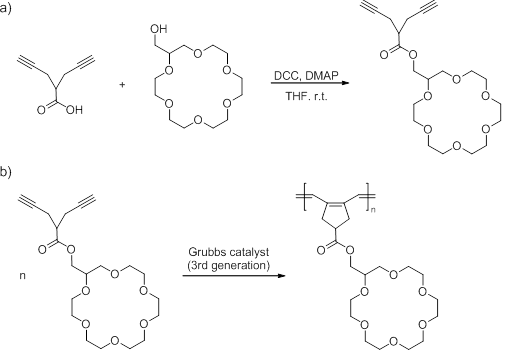
A diyne monomer with 18-crown-6 ring was synthesized by the esterification reaction of 2-(2-propynyl)-4-pentynoic acid with 2-hydroxymethyl-18-crown-6 catalyzed by dicyclohexylcarbodiimide (DCC) and 4-dimethylaminopyridine (DMAP) in THF at room temperature (Scheme 1a). After optimizing the reaction conditions, the yield of the diyne monomer (relative to 2-hydroxymethyl-18-crown-6) can reach 78%. Polymerization of the diyne monomer was studied using the 3rd-generation Grubbs catalyst as initiator (Scheme 1b). A variety of polymerization conditions with varied feed ratios of monomer to initiator ([M]0:[I]0) were investigated. Polymerizations were quenched with ethyl vinyl ether when the polymerization times (up to 1 hour) were reached. Then quenched polymerization solutions were analyzed by 1H NMR spectroscopy to determine monomer conversions (up to ~80%). Polymers were recovered from the quenched polymerization solutions by precipitation in methanol, followed by washing with hexane and ethyl acetate.
Scheme 1
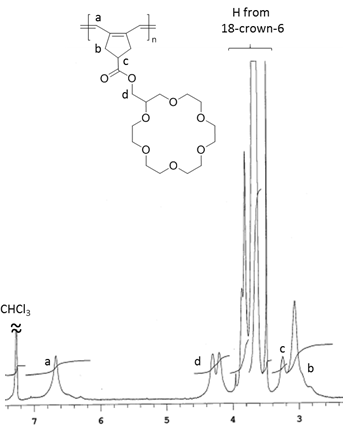
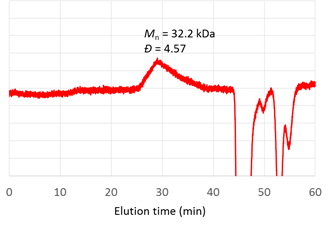
Polymers were further characterized by 1H NMR spectroscopy and gel permeation chromatography (GPC). 1H NMR analysis verified that polyrings were synthesized by metathesis polymerization of the diyne monomer. 1H NMR spectrum of a representative sample of polyrings (obtained from a trial with ([M]0:[I]0 = 50, by 1 h of polymerization in THF at 0 °C) is shown in Figure 1. However, GPC analysis indicated that the polymerization behavior was not well-controlled. DMF GPC analysis of these samples showed broad GPC peaks, corresponding to high MW dispersity (Ɖ). For instance, the representative sample had a number-average MW (Mn) of 32.2 kDa and a Ɖ of 4.57 relative to linear polystyrenes (Figure 2). Analysis of these samples with THF GPC was also attempted. However, even broader, multi-mode GPC curves were obtained for these samples by THF GPC, presumably due to unfavorable interactions of the polymers with GPC columns in THF. Because previously we demonstrated that metathesis of a diyne monomer with cycloalkane ring yielded well-defined polymers, the polymerization results from the systems using crown ether-based diyne monomers suggest that the 18-crown-6 moieties might interact with ruthenium-based chain-growth centers during polymerization, resulting in complicated and uncontrolled polymerization behavior. Restriction of the interaction of crown ether moieties with ruthenium-based species might be a valid strategy to enhance the overall polymerization control and to achieve well-defined polymers. However, because of time limitation, systematic study on enhancement of polymerization control by restricting such interaction has not been conducted.
Figure 1. 500 mHz 1H NMR spectrum of a representative sample of polyrings with 18-crown-6 moieties (in CDCl3).
Figure 2. GPC analysis of a representative polyring sample (eluent: DMF).
In summary, synthesis and polymerization of a diyne monomer with 18-crown-6 ring was studied in the past grant year. High yield (i.e. 78%) in the esterification synthesis of the monomer was achieved. Metathesis of the monomer yielded polyrings with each monomer unit carrying a 18-crown-6 moiety. However, as indicated by the relatively broad MW dispersity of the polyrings, the polymerization was not well-controlled, and therefore, the synthesis of block copolymers was not attempted at this stage. A specific strategy to enhance polymerization control is proposed. Although the synthesis of well-defined OEG-based polyrings still remains challenging, the results obtained from this project serve as a valid basis for future advances to achieve these novel model polymers.