Reports: DNI655672-DNI6: Chemical Kinetics of Gasoline Mixtures under Negative Valve Overlap Reforming Conditions
Subith Vasu, PhD, University of Central Florida
Summary:
During combustion, fuels (e.g., gasoline) breakdown into intermediates via pyrolysis and oxidation channels, thus the formation and consumption time-scales of intermediates play crucial role in heat release and combustion. Chemical kinetics investigation of gasoline-relevant fuels and intermediates are carried out in this study using variety of combustion fundamental experiments. Below we discuss some of the findings from our study.
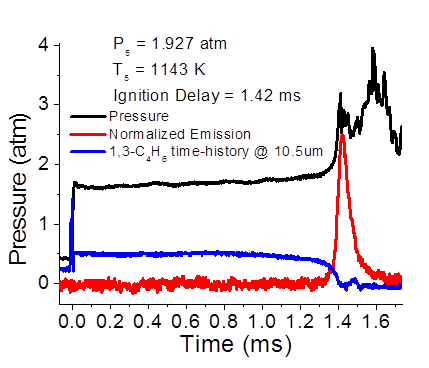
The chemical kinetics of 1,3-butadiene (1,3-C4H6) is important because it is a major intermediate during combustion of real fuels. However, there is only limited information on the chemical kinetics of 1,3-butadiene combustion, which has applications in several combustion schemes that are currently being developed, including spark-assisted homogeneous charge compression ignition and fuel reformate exhaust gas recirculation. In order to improve the accuracy of chemical kinetic models, the ignition delay times of 1,3-butadiene mixtures has been investigated using pressure traces (Fig. 1). Oxidation of 1,3-butadiene/oxygen/argon mixtures at equivalence ratios, Ф = 0.3 behind reflected shock waves has been studied at temperatures ranging from 700 to 1800K and at pressures ranging from 1 to 3atm. Reaction progress was monitored by recording concentration time-histories of 1,3-butadiene (1,3-C4H6) and OH* radical at a location 2cm from the endwall of a 13.4m long shock tube with a 14cm inner diameter. 1,3-C4H6 concentration time-histories were measured absorption spectroscopy at 10.5μm from the P14 line of a tunable CO2 gas laser. The reference and transmitted intensities of each laser were recorded with thermoelectrically cooled mercury cadmium telluride photovoltaic detectors. In order to obtain more accurate measurements, a differential absorption scheme was employed to remove contributions to the absorption from interfering species. In this method, a peak and valley wavelength pair is selected such that the difference in absorption cross section at the two wavelengths is maximized for the species of interest and minimized for interfering species. At the selected wavelength pair, the most abundant interfering species have an approximately constant absorption cross section, and thus contribute equally to the absorbance at each wavelength. By subtracting the measured absorbance at the peak and valley wavelengths, this contribution from interfering species is canceled out, leaving signal due only to the species of interest and thus minimizing noise in the measurement. OH* production was measured by recording emission around 307nm with a preamplified gallium phosphide detector and a bandpass filter. Ignition delay times were also determined from the OH concentration time-histories. The measured concentration time-histories and ignition delay times were compared with literature data and modeled predictions from literature kinetic models. The measured time-histories and ignition delay times provide targets for the refinement of chemical kinetic models at the studied conditions, which will advance the development of new engine technologies.
Figure 1. An example of ignition trace in 1,3-butadiene (1%)/O2 (18.3%)/Ar (80.7%) mixture.
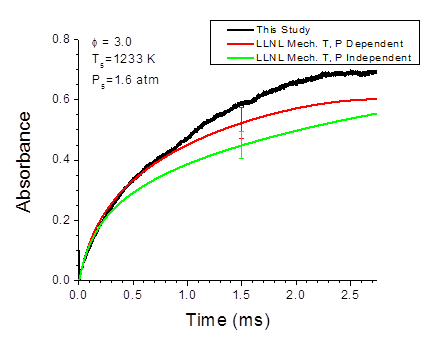
The chemical kinetics of n-heptane (n-C7H16) – an important reference compound for real fuels – oxidation are well studied at stoichiometric and lean conditions. However, there is only limited information on the n-heptane chemical kinetics at fuel-rich combustion. In order to verify the accuracy of chemical kinetic models at these conditions, the oxidation of rich n-heptane mixtures has been investigated. Combustion of n-C7H16/O2/Ar mixtures at equivalence ratios, Ф, of 2.0 and 3.0 behind reflected shock waves has been studied at temperatures ranging from 1066 to 1502 K and at pressures ranging from 1.4 to 6.2 atm. Reaction progress was monitored by recording pressure, and absorption time-histories of ethylene (C2H4) and n-heptane at a location 2 cm from the endwall of a 14-cm inner diameter shock tube. Ethylene and n-heptane absorption time-histories were measured, respectively, using absorption spectroscopy at 10.532 μm from a tunable CO2 laser and at around 3.4 μm from a continuous wave distributed feedback interband cascade laser (ICL). The measured absorption time-histories were compared with modeled predictions from the Lawrence Livermore National Lab (LLNL) detailed n-heptane reaction mechanism. An example ethylene time-trace during heptane combustion is shown in Fig. 2.
Figure 2. Absorbance time-histories for ethylene (2%). ϕ = 3, P ≅ 1.5 atm. Simulations were performed using the detailed LLNL n-heptane mechanism with Chemkin Pro.
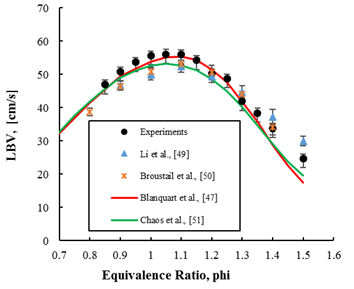
The laminar burning velocity (LBV) is defined as the velocity at which unburned gases moves through the combustion wave in the direction normal to the wave surface. The LBV is not only important in understanding several aspects of combustion in spark ignition engines, and fuel comparisons; it is commonly used to validate the chemical kinetic schemes.
Figure 3: Laminar burning velocity (cm/s) vs Φ for iso-octane (C8H18)-Air mixture (current study) at initial temperature of 393K and initial pressure of 1 atm. Iso-octane is an important reference fuel for gasoline. Comparisons with literature data (Li et al. and Broustail et al.) and predictions of kinetic mechanisms (Blanquart et al. and Chaos et al.) are also shown.
Impact of Research and ACS PRF Funding:
We provided the first high temperature species concentration time-histories during heptane and butadiene oxidation and improvements to kinetic mechanisms were suggested. The detailed interpretation of the experimental data will also serve as validation targets for quantum chemical calculations of the potential energy surfaces for these important reaction systems.
The ACS PRF DNI grant has had very significant impact on the PI and both graduate and undergraduate students. The PI was able to recruit and train students for research, as well as publish several journal and conference articles based on this work. These publications will provide professional recognition for the PI while establishing his career. During this reporting period (Aug 2016-Sept 2017), the grant partially supported 3 graduate students and introduced two undergraduate students to research (some of them are continuing for graduate studies with the PI). Also, the broader impact of the grant is that it helped several underrepresented students to pursue education and research. One of the graduate students (Joseph Lopez) who is Hispanic was able to obtain full time job as an engineer at the Air Force Research Laboratory. Another undergraduate student (Erik Ninnemann) published a paper in the prestigious Combustion and Flame journal.