Reports: DNI557055-DNI5: Catalytic C-C Bond Formation to Synthesize Carboxylic Acids Using CO2
Kara Stowers, PhD, Brigham Young University
The primary objective of this work is to demonstrate selective and efficient C-C bond development on metal surfaces, incorporating CO2 into the newly formed molecule. Single crystal Cu(110) was studied with the ultra-high vacuum technique temperature programmed reaction spectroscopy. X-ray photoelectron spectroscopy will be used for determining intermediates at the surface and these data combined will be used to elucidate the mechanisms of reactivity.
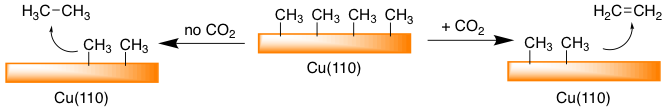
We have found preliminary results that CO2 can promote different reaction selectivity without forming new bonds with the reagents (Scheme 1). These reactions can occur through an intermediate carboxylate that forms on the surface and then a homocoupling of fragments. Alternatively, CO2 can react with the surface through dissociation, releasing adsorbed O to the surface that can then act as an oxidant to enact transformations. We are interested in the scope of reactions between adsorbed fragments that are influenced by the behavior of CO2 dosing, and to understand the differences in reactivity between CO2 and O2 in terms of redox behavior. Currently there is a number of hydrocarbon upgrading reactions being studied for which CO2 is considered an oxidant.
|
Scheme 1. Selectivity differences are observed for methyl fragments on a Cu(110) dependent on CO2. |
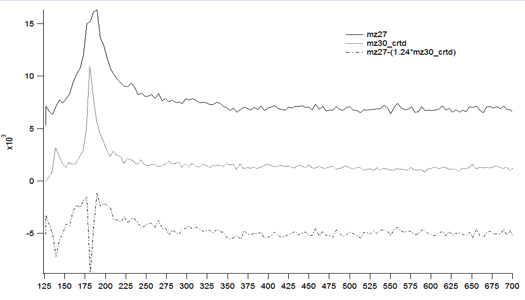
Initially, the bulk of the work accomplished was to get the chamber up and running and to identify the baselines for controls and fragmentation. We have used the studies done by Bent and coworkers regarding the reactivity of iododmethane on a Cu(110) surface in order to study the reactivity of CO2 in the presence of nucleophilic surface bound molecules. The m/z ratios are chosen in order to delineate between ethane and ethylene formation. Figure 1 shows that for iodomethane dosed at low temperatures, ethane evolves from the surface as the major product with minor contributions from ethylene. This is consistent with reports previously published where high exposures of CH3I selectively form more ethane than methane or ethylene.
|
Figure 1. Ethane is the major product from the homocoupling on methyl fragments on a Cu(110) surface. Conditions: 6L of CH3I at 126K, TPD 5.7 K/s
|
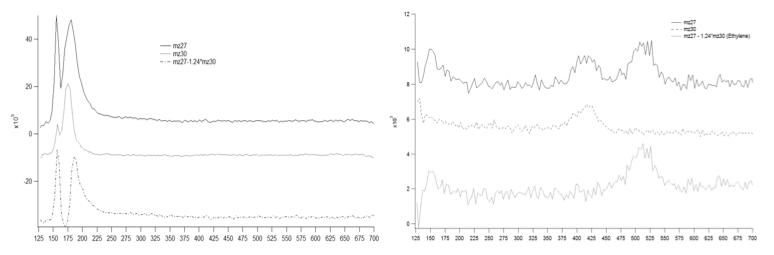
At low temperatures in the presence of dosed CO2, the selectivity changes to now evolve both ethane and ethylene from the surface as shown in Figure 2a. Also it is observed that ethylene desorbs at a lower temperature than ethane. At high temperature, dosing CH3I without CO2 leads again to only ethane with little ethylene. Alternately, dosed iodomethane at high temperature now exposed to CO2 shows ethylene formation with evolution of the products at different temperatures as shown in Figure 2b. These results are similar to what was observed for low temperature experiments. Ethane evolves at 490-540K while ethylene evolves from 375-425K. There is also a difference in the desorption temperatures of the evolved products. These results require further study using XPS analysis of the intermediates that form at the surface and an identification of what intermediates are formed under each condition to obtain the same products.
|
Figure 2. In the presence of CO2, ethylene is now formed in addition to ethane from a methyl fragment covered Cu(110) surface. a) conditions: 6L of CH3I at 129K, 1L CO2 at 128K b) conditions: 4.5L CH3I at 300K, 2L CO2 at 130K; TPD 3.4K/Sec
|
We next began to investigate the cross-coupling between alkyl fragments (Scheme 2). These findings are relevant to hydrocarbon isomerizations and sigma-bond metathesis reactions at high temperature on Cu surfaces. For example, the homo-coupling of alkyl fragments on the surface of Cu(110) has been demonstrated for methyl and propyl groups to form ethane and hexane respectively. We must first understand the reactivity of alkyl fragments in the absence of CO2 prior to understanding and characterizing the reactivity in the presence of CO2. The difference from the other metals with copper is that the alkyl structure and substituents have a large effect on reactivity whereas the reaction peak temperature varies from 155K to 320K across the substituent scope. The reactivity of fragments with β-hydrogens on Cu surfaces is found to occur at fairly low temperatures (230-250 K). Tuning reactivity with desorption can help us have a handle for identifying how they might react with CO2.
|
Scheme 2. Preferential cross-coupling is observed between methyl and propyl groups on a Cu(110) surface.
|
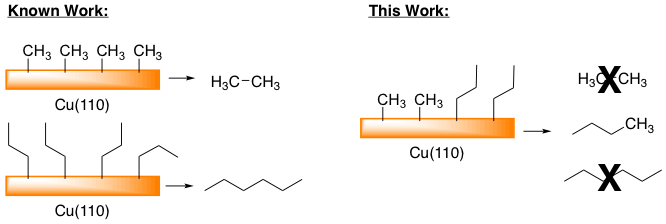
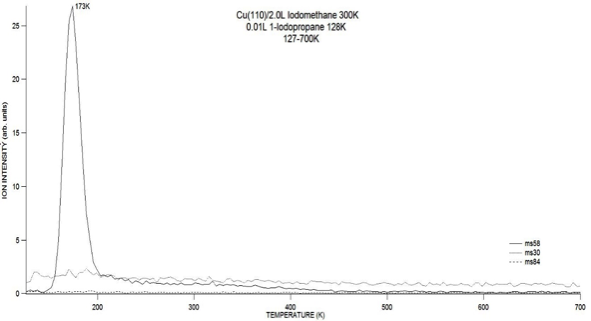
We chose to look at the C1 and C3 fragments so as to selectively see m/z ratios for a C4 product upon cross-coupling compared to the C2 and C6 that are formed for homo-coupling. As shown in Figure 3 these two fragments co-adsorbed to a Cu(110) surface appear to selectively cross couple with each other to form butane exclusively. Iodopropane was physisorbed to a pre-dosed surface at a temperature of 127K, while iodomethane was dosed to the surface at 300 K resulting in chemisorption through the dissociation of the C-I bond. Therefore, the selectivity may be due to ordering of the fragments on the surface but further investigation of the selectivity is in process.
|
Figure 3. Propyl fragments and methyl fragments react preferentially on a Cu(110) surface. No homo-coupling products are observed. Conditions: 0.01L C3H7I at 127K; 2L CH3I at 300K; TPD 3.4 K/s
|
In order to fully understand the reactivity of fragments for coupling reactions and reactions that incorporate CO2 we will need to continue to study the order and temperatures of dosing to determine selectivity. The impact of this research allows us to determine the preference for coupling between fragments. The coupling preferences will underline the activation energy for desorption and the nucleophilicity of the fragments at the surface in order to then determine how to design new reactions for C-C bond formation with CO2. We have included training two graduate students, one undergraduate, and one post-doc on this project. The skills that they have learned from analysis of the experiments, and the design of controls will be impactful for their careers as scientists studying catalysis.