Reports: DNI954904-DNI9: Probing Relationships between Carbon Nanostructure and Binary Molecular Mobility in "Crowded" Nanoporous Spaces
Ryan Lively, Georgia Institute of Technology
Membrane-based processes can reduce the carbon- and energy-intensity of traditional thermal separations such as distillation and crystallization that require expensive phase change steps. The supply chains of many polymers, plastics, fibers, solvents and fuel additives depend on benzene, a cyclic hydrocarbon, as well as on its derivatives such as mesitylene and the xylene isomers. Carbon molecular sieve (CMS) membranes are promising materials for challenging solvent separations. Indeed, CMS materials possess excellent thermal and chemical stability, even in aggressive solvent environments.
The major goals for this reporting period were (1) to synthesize high quality polymer of intrinsic microporosity-1 (PIM-1) that readily serve as the polymer precursors and (2) to fabricate defect-free CMS dense membranes for organic solvent separations.
Accomplishments
Synthesis of PIM-1
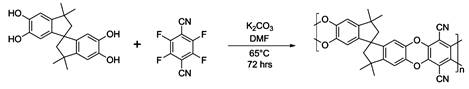
PIM-1 was synthesized using the low temperature polycondensation method developed by Mason and Budd as shown in Figure 1. 5,5’,6,6’-Tetrahydroxy- 3,3,3’,3’ -tetramethyl-1,1’-spirobisindane (TTSBI) was purified by reprecipitation from hot methanol with dichloromethane, and tetrafluoroterephthalonitrile (TFTPN) was recrystallized via vacuum sublimation at 140 °C. The two purified monomers were then added into anhydrous DMF with a 1:1 molar ratio in a round-bottom flask. After the monomers were completely dissolved, anhydrous potassium carbonate was added to the solution, and the polymerization reaction was continuously stirred under a nitrogen atmosphere at 65 °C for 3 days. After the reaction, upon cooling, DI water was used to quench the reaction and precipitate the PIM-1 polymer. The polymer was then collected by filtration and washed with additional DI water to remove residual salts. Repeated reprecipitation from choloroform gave us purified polymer. Finally, the yellow PIM-1 was dried in vacuum oven under 70 °C overnight before use.
Figure 1. Reaction scheme for the low temperature synthesis of PIM-1.
Fabrication of Polymeric Dense Films
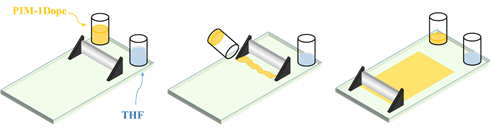
Polymeric dense films were cast using a blade within a sealed glove bag kept in a fume hood as shown in Figure 2. Before dissolution in THF, PIM-1 was dried in the vacuum oven for at least 12 hours to remove any moisture. The PIM-1 and THF solution was then placed on a roller for at least 6 hours to form a homogeneous solution, which is called the dope here. After being purged with nitrogen three times, the glove bag was fully saturated with THF. Afterwards, the dope was transferred from the vial to glass plate and then cast into a film. Subsequently, the film will solidify as the THF slowly evaporated from the solution for 1 days, followed by vacuum drying for another 24 hours. With no extra supports, defect-free PIM-1 homogeneous dense films were successfully fabricated.
Figure 2. Schematic diagram of the homogeneous dense films casting process.
Fabrication of CMS Dense Films
 |
CMS dense films were produced by pyrolyzing the PIM- dense films under an argon atmosphere in a pyrolysis set-up. Dried circular PIM-1 polymeric films were first placed on a mesh plate and then put into a quartz tube before being loaded into a three zones pyrolysis furnace. The quartz tube was sealed with a pair of SS 304 vacuum flanges with double high-temperature silicone O-rings. An inert atmosphere was achieved by purging the tube with argon for at least 12 hours, and the typical oxygen concentration was below 1 ppm as measured by an oxygen analyzer.
Figure 3. Photographs of the homogeneous dense membranes: (a) PIM-1, (b) CMS derived from PIM-1.
Organic Solvent Forward Osmosis Test
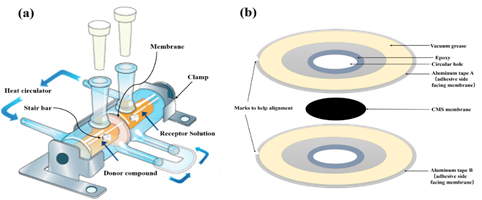
The CMS dense membranes were tested for organic solvent forward osmosis using a two side-by-side cell as described in Figure 4. Briefly, a circular CMS membrane was masked by sandwiching the membrane between two impermeable aluminum tapes and then mounted into a cell to run permeation measurements. For sealing purposes, epoxy was applied at the interface of the CMS membrane and aluminum tape while vacuum grease was applied at the adjacent interface between the clamping surface and aluminum tape. Pure mesitylene was chosen as the initial compound in the “draw” reservoir.
Figure 4. (a), Side-by-side diffusion cell used for OSFO experiments and (b), Schematic representation of assembling a CMS sandwich membrane.
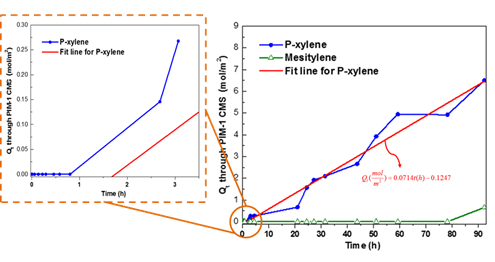
The plots of Qt versus time t for organic solvent passing through the PIM-1 CMS membrane pyrolyzed at 650 oC is shown in Figure 5. The mole fraction of p-xylene in the “draw” reservoir during the measurement is always less than 0.001.
Figure 5. Qt plot for p-xylene and mesitylene passing through PIM-1 CMS membrane pyrolyzed at 650 oC.
By fitting the experimental data, the diffusion coefficient and permeability of p-xylene through CMS membrane can be calculated as shown in Table 1.
Table 1. OSFO results for p-xylene and mesitylene through PIM-1 CMS-650 oC.
Items |
PIM-1 CMS-650 oC |
Lag time Ɵ (hours) |
1.74 |
Film thickness (µm) |
18.7 0 |
Diffusion coefficient (cm2/s) |
9.304×10-11 |
Permeability (mol.m/(m2.s.Pa)) |
3.28×10-13 |
The permeability of p-xylene through Torlon 4000T membrane was also tested by Chafin et al. using a pervaporation apparatus at temperatures in excess of 200oC. The xylene pervaporation in annealed Torlon 4000T film at 200oC gave a permeability of 0.25 Barrer, 8×10-17 mol.m/(m2.s.Pa), which is lower than the permeability of the CMS membranes tested here. The diffusion coefficient of mesitylene through a PIM-1 CMS membrane pyrolyzed at 650 oC was calculated to be 1.75×10-12 cm2/s with a lag time of 92.25 hours. A diffusion selectivity of 53 was achieved for p-xylene over mesitylene through the PIM-1 CMS membrane. These results show that CMS membranes have the potential to serve as selective OSRO membranes.
Conclusion
CMS membranes are produced by the pyrolysis of PIM-1 using a specialized thermal program and an inert pyrolysis atmosphere that results in the decomposition of the polymer precursor into carbon sheets. Cumulative concentration profiles analysed by time lag method reveal that, for a CMS membrane pyrolyzed at 650 oC, the permeability for p-xylene is 3.28×10-13mol.m.m-2.s-1.pa-1 and p-xylene/mesitylene diffusivity selectivity is 53. The project describes the process of synthesizing PIM-1, a novel class of microporous polymer, fabricating CMS membranes and testing the resulting membrane. Overall, this study provides insight into the design of microporous CMS membranes for organic solvent separations.