Reports: DNI356568-DNI3: Nickel-Mediated Bimolecular Bond Formation and the Applications in the Conversion of Petroleum Products
Tianning Diao, PhD, New York University
With the support of ACS-PRF, our research group has made significant progress in the fields of Ni catalyzed organic transformations and the fundamental mechanism of Ni-mediated bond formation.
1. Reductive Cyclization of Dienes
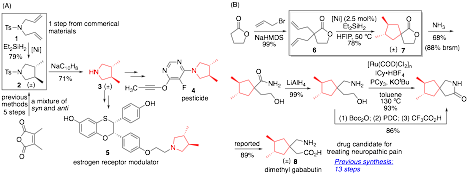
Carbocycles and heterocycles with trans-vicinal disubstituents are commonplace in chemical and pharmaceutical products, but limitations in current synthetic methods have restricted efficient access to these molecules. We developed a Ni(I)-catalyzed reductive cyclization reaction for preparing trans-disubstituted cyclic molecules, where common Ni(0) and Ni(II) precursors failed to afford desirable results (Scheme 1). This reductive coupling reaction has found appealing applications in preparing bioactive molecules. The trans-dimethyl-pyrrolidine product 2 can be readily deprotected to generate trans-3,4-dimethylpyrrolidine 3, which serves as the precursor to pesticide 4 and a selective estrogen receptor modulator 5 (Scheme 2A). Previous methods to prepare the same product require multiple steps from the commercially-available dimethylmaleic anhydride, and give a mixture of cis- and trans-diastereomers. The gababutin derivative 8 exhibits efficacy towards relieving neuropathic pain and anxiety in in vivo models. In contrast to the previous synthesis, which required 13 steps for a 14% overall yield, our reductive cyclization constructed the 3,4-dimethyl cyclopentane motif and afforded 8 in 6 steps and 42% overall yield.
Scheme 1. Ni(I)-Catalyzed Reductive Cyclization of Dienes
Scheme 2. Applications of Ni(I)-Catalyzed Reductive Cyclization of Dienes
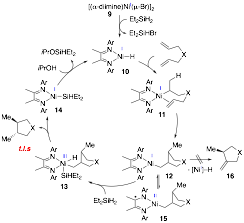
Our mechanistic studies reveal that the reaction proceeds via two-electron organometallic pathways on a paramagnetic platform consisting of Ni(I) and Ni(III) intermediates (Scheme 3). This mechanism is distinct from single-electron transfer pathways, which are extensively proposed in Ni-catalyzed cross-coupling reactions, as well as from mechanisms mediated by Ni(0) and Ni(II). The Ni intermediate 12 in the +1 oxidation state is stabilized by the redox-active α-diimine ligand. The electron-rich, formal Ni(I) intermediate 12 undergoes rapid oxidative addition with Et2SiH2, which outcompetes β-H elimination to form the unsaturated cycloisomerization product 16. This chemoselectivity sharply distinguishes the Ni(I) catalyst from previous Ni(II) catalysts, which favor β-H elimination rather than reaction with silanes. The two-electron organometallic pathway accounts for formation of the trans-product, whereas cyclizations proceeding through radical intermediates give a mixture of cis and trans products. The novel mechanism provides opportunities to design Ni-catalyzed reactions in the absence of organic radical intermediates, allowing for unique selectivity to be achieved due to the electronic properties of Ni(I) and Ni(III) intermediates.
Scheme 3. Proposed Mechanism of Ni(I)-Catalyzed Reductive Cyclization of 1,6-Dienes
2. Structure and Isotope Effects of β-H Agostic (a-Diimine)Ni Cation as the Polymerization Intermediate
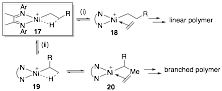
Cationic (α-diimine)Ni complexes and derivatives, pioneered by Brookhart and coworkers, represent important polymerization and oligomerization catalysts. The catalysts are particularly valuable with readily modifiable ligand structures to access different molecular weights and morphologies. Previous mechanistic studies identified the β-agostic Ni complex 17 as a key intermediate in polymerization (Scheme 4). The β-agostic Ni complex 17 is the catalyst resting-state for polymerization of a-olefins, such as propylene (step i). Reversible β-H elimination from the β-agostic Ni 17 followed by hydride re-insertion (step ii) leads to branched polymers. As a result, the stability of the β-agostic Ni complex towards olefin coordination and β-H elimination influences the molecular weight and linear/branched ratio, thus the morphology, of the polymer. Brookhart and coworkers characterized β-agostic (α-diimine)Ni complexes by 1H NMR spectroscopy. But no X-ray structure has been obtained previously for the highly reactive polymerization intermediates.
Scheme 4. Mechanism of (a-diimine)Ni-Catalyzed Polymerization of Ethylene
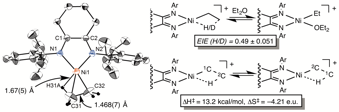
Fundamental interest in transition metal β-agostic bonds, in combination with their critical roles in catalysis, encouraged us to isolate and obtain the single-crystal X-ray characterization of cationic (α-diimine)Ni-ethyl and isopropyl β-agostic complexes. The sharp Ni-C_-C_ bond angles (75.0(3)¼ and 74.57(18)¼) and short C_-C_ distances (1.468(7) and 1.487(5) ) of the (α-diimine)Ni-ethyl compound provide unambiguous evidence for β-agostic interaction. An inverse equilibrium isotope effect (EIE) for ligand coordination upon cleavage of the agostic bond highlights the weaker bond strength of Ni-H relative to the C-H bond. An Eyring plot for β-hydride elimination-olefin rotation-reinsertion is constructed from variable-temperature NMR spectra with 13C-labeled agostic complexes. The enthalpy of activation (DHà) for β-H elimination is 13.2 kcal/mol. These results offer important mechanistic insight into two critical steps in polymerization: ligand association upon cleavage of the β-agostic bonds and chain-migration via β-H elimination.
Scheme 5. Single-Crystal X-ray Structure of Ni β-Agostic Complex and the Kinetic Barriers for β-H Elimination