Reports: ND356027-ND3: Highly Active Organometallic Complexes Obtained through Mechanochemical Synthesis
Timothy P. Hanusa, Vanderbilt University
PROGRESS REPORT
General Goals and Impact: The general aim of the grant is to explore the use of mechanochemical methods of synthesis (e.g., with grinding and milling) in the preparation of new organometallic compounds that may display high reactivity and/or catalytic activity. Such solid-state reactions provide the opportunity to investigate compounds not attainable when a solvent is present, either because useable solvents interfere with the interaction of the reagents, or because solvent molecules could bind strongly to the product and change its structure and reactivity. It is also a type of "green" chemistry, which complements the use of earth-abundant metals in synthesis. The general paucity of information about organometallic mechanochemistry ensures that there will be important discoveries to be had with a variety of ligands and with metals from across the periodic table.
The results achieved to date have had a major positive impact on the direction of my research. Mechanochemical synthesis has attracted the interest of both potential graduate students and undergraduate researchers. One first-year graduate student, who had intended to work in traditional organic areas, ended up joining my research group once he learned about the possibilities for mechanochemical organometallic synthesis. One senior graduate student was supported on the ACS-PRF grant starting in the summer of 2016. He defended his Ph.D. dissertation, which incorporated a substantial amount of mechanochemical work, in June 2017. An undergraduate presented the results of his mechanochemical research at the Fall 2017 ACS National Meeting in Washington, DC. An NSF proposal was submitted that incorporated results from the ACS-PRF grant, and it was subsequently approved for funding. It is fair to say that the stimulus provided by the New Directions grant has been substantial and far-reaching.
Research Results
1. Complexes with substituted allyl ligands
(a) s-Block allyl complexes: new ligand conformations and reactivity.
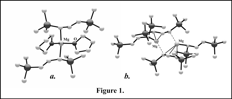
Mechanochemistry was used to address the synthesis of magnesium allyl compounds with bulky allyl ligands (A'= 1,3-(SiMe3)2C3H3). The compounds were already known from solution syntheses, in both solvated and unsolvated forms (Figure 1, and eq. 1, 2).
Without coordinated THF, the monomeric MgA'2(thf)2 complex (Figure 1a) is isolated as the dimeric {MgA'2}2 (Figure 1b). More critically, although the solvated complex is completely inactive as a catalyst initiator, preliminary data has indicated that the unsolvated dimer is modestly active for methyl methacrylate polymerization (TOF = 3,000 h-1).
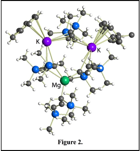
We attempted to use mechanochemistry to form the unsolvated {MgA'2}2 directly from MgBr2 and K[A']. This led to unexpected results, i.e., ball milling of MgBr2 and 2 equiv. of K[A'], extraction of the ground mixture with hexanes, and recrystallization of the product from toluene produced the heterotrimetallic species (A')Mg(µ-A')2K(h6-tol)(µ-A')K(h6-tol) (Figure 2). This is a product never observed from solution synthesis; it formally represents the product of 4 equiv. of K[A'] with MgBr2 (eq. 3), even though that was not the ratio present at the start of the reaction.
The presence of both Mg and K in the allyl complex reflects the fact that the reaction takes place in a single phase, and not in a system where the anticipated KBr byproduct would be removed from the reaction medium by precipitation. Tests of this compound and a related calcium species for polymerization of methyl methacrylate are pending.
(b) Catalysis with a mechanochemically generated aluminum allyl
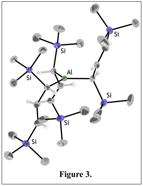
The ball milling AlCl3 with 3 equiv. of K[A'] yields AlA'3 (Figure 3), the first example of an unsolvated tris(allyl)aluminum complex. In conjunction with the research group of Prof. Brian Long, of the Univ. of Tennessee, Knoxville, we have found that AlA'3 polymerizes lactide, producing a Mw (52,000 g mol-1) about 200% higher than the standard aluminum catalyst Al(OiPr)3 and 40% greater than AlMe3. It is still not as active as could be desired, and the polydispersity of the polymer (PDI = 3.0) needs improvement. We are addressing these issues with the production of differently substituted allyl ligands that may enhance the reactivity and provide better control over chain length.
(c) Non-stoichiometry in beryllium chemistry
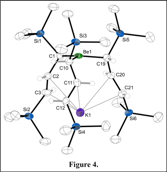
Owing to concerns about toxicity, the organometallic chemistry of the lightest alkaline-earth metal, beryllium, is relatively understudied. In an attempt to form the solvent-free "BeA'2", K[A'] and BeCl2 were milled together without solvent; unexpectedly, the beryllate complex K[BeA'3] was the only isolable product (Figure 4). Remarkably, the same product is formed regardless of whether the ratio of K[A'] to BeCl2 was 1:1, 2:1, or the ideal 3:1. Calculations suggest that a "BeA'2" complex, should it be forming, would be coordinately undersaturated; reaction with nearby K[A'] to generate the beryllate evidently occurs to fill the coordination sphere. These results highlight the opportunities to isolate kinetic products that would not be observed from solution chemistry, a possibility that should extend to other metals.
2. Non-stoichiometry in transition metal chemistry
The shift in product distribution in reactions when conducted mechanochemically compared to solution routes is observed in transition metal chemistry as well, and is not confined to allyl complexes. The reaction of Cp2TiCl2 with potassium tert-butoxide produces four different products when conducted with ball milling (Figure 5). Counterintuitively, the major product is CpTi(OtBu)3 (47%), despite the less than optimum stoichiometric ratios of reagents. When the same reaction is conducted in THF, the majority product is the expected Cp2Ti(OtBu)2 (70%), with CpTi(OtBu)3 present as only a minor by-product (13%). Mechanochemical activation completes the reaction in far shorter time than is the case in solution (15 min vs. 8 hours, respectively). The ring displacement reactions are a feature only of the titanium-based chemistry; when the same reactions are conducted with Cp2ZrCl2 or Cp2HfCl2, only Cp2MCl(OtBu) or Cp2M(OtBu)2 are formed.
The experience we have already gained with the mechanochemical synthesis of organometallic compounds will be extended to new research. The accessibility of kinetically preferred compounds is likely to be a key feature in the synthesis of currently unknown compounds and catalysts.