Reports: DNI556018-DNI5: Investigating the Impact of Strain on Electrochemical CO2 Reduction Using Model Bulk Metallic Glass Materials
David P. Fenning, PhD, University of California, San Diego
In the first year of this project, we have made significant progress toward the scientific and educational goals. We are studying CO2 utilization for on-demand industrial precursor production using an electrochemical reduction pathway. In CO2 electrocatalysis, the principle challenge is the lack of catalysts that are active and selective for particular products. The multitude of intermediates in the CO2 reduction reaction leads to poor selectivity, and the large uphill thermodynamics of key elementary steps means that significant energetic losses are incurred in converting CO2 to C1-C3 hydrocarbons or oxygenates. In a new approach to address this catalysis challenge, in this work we are seeking to study surface strain as an analog, easily-controllable variable to tuning catalytic selectivity and activity using model materials that can sustain high elastic strains.
One of the barriers to conducting experiments in CO2 electroreduction is the relative difficulty of the experimental setup. Careful attention must be paid to fully capture and analyze all reaction products in order to be able to attribute Faradaic current appropriately. It is largely meaningless to study the CO2 reduction reaction without detailed product analysis since one of the main parasitic (but dominant) reactions is hydrogen evolution. Thus, a rigorous analytical chemistry component comes in addition to catalysis synthesis and routine electrochemical measurement in pursuing questions regarding electrolysis of CO2.
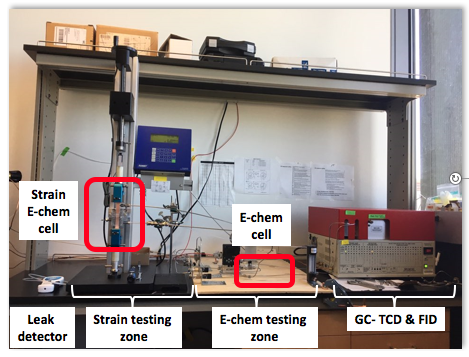
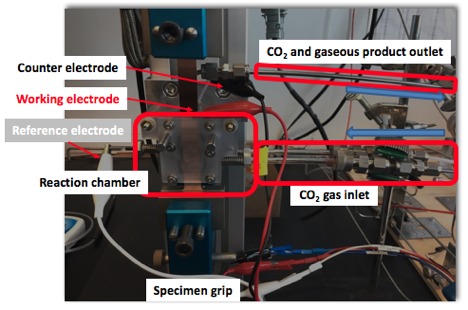
We have established an integrated in situ electrochemical test station where we can apply various stress states to the sample, while the electrochemical reaction is still carefully contained to collect all products of reaction for chemical analysis. We have validated our chemical detection, calibrated our samples under tensile stress, and are prepared to launch the first analysis of the strain dependence of catalytic activity and selectivity for the CO2 reduction reaction as we enter the second year of this project.
Impact on Students who Participated in the Project
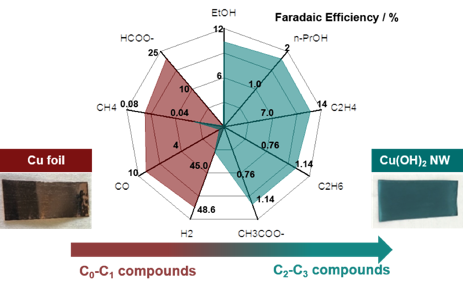
The students who have participated in the project have had to learn the exquisite attention to detail and careful experimental process necessary to achieve robust CO2 reduction experimental results. During this first year, we have been able to baseline our setup against the state-of-the-art results in literature, achieving chemical detection at or better than the most prominent reports on canonical planar copper catalysts. Using synthetic methods they learned in our Nanonengineering curriculum, we turned to a high-surface area, defective nanowire catalyst for our first tests of the validated system. The results were promising and are under preparation for publication.
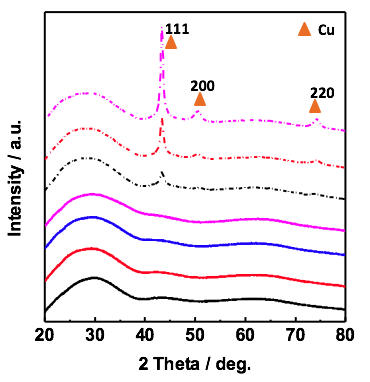
The principle scientific hypothesis of the project requires testing of model metallic glasses under strain during electrolysis. Metallic glasses can sustain high elastic strains, much larger than typical metallic materials. We have successfully produced metallic glasses via sputtering and have confirmed their amorphous nature via X-ray diffraction and electron beam backscattered diffraction. The students on the project have thus established not only analytical chemistry and basic electrochemistry experimental skills, but also physical vapor deposition and top down fabrication experience as well as materials science characterization.
The undergraduate students who participated in the work were introduced to laboratory basics, chemical synthesis, and electron microscopy characterization. The two undergraduates excelled. They presented their work related to this project at the ACS Student Association undergraduate research symposium and will be co-authors on the above mentioned publication.
Impact on My Career
The support of the DNI award has been absolutely fundamental in providing a runway for me to establish my laboratory's investigations in electrocatalysis. Since CO2 reduction is a new direction in my research agenda, I would not have been able to tolerate the extensive experimental apparatus development that is necessary to test the hypothesis we are after. Now that we have it established, interesting results are opening up new avenues for investigation. We are eager to broaden our scope and have easily many years of investigation ahead of us. This award will almost without a doubt thus provide the foundation for a line of research for years to come within my group and a time- and resource-bridge to establish myself in the field. Truly, it has been a catalyst, bringing down the barrier for me to pursue this important research.
Fig. 1 The CO2 reduction electrochemical test station with in-situ strain application and integrated product analysis.
Fig. 2 Close up of the in situ strain electrochemical cell with product analysis, designed, constructed, calibrated, validated, and ready for operation.
Fig. 3 Metallic glass films containing copper as a major component (bottom, solid lines) show only a diffuse background of scattering. Pure copper films (top, dotted lines) show crystalline peaks.
Fig. 4 In checking out the electrochemical cell (no strain), using a nanowire Cu-based electrode fabricated in the lab, we have demonstrated a dramatic shift from C0 and C1 products on planar copper to predominantly C2-C3 products. Results are under preparation for publication.