Reports: ND155514-ND1: Microwave-Promoted Tin-Free and Initiator-Free Cyclizations of Iminyl and Aminyl Radicals: A Convenient New Method of Synthesizing Complex Amines from Simple Precursors
Steven L. Castle, Brigham Young University
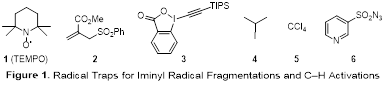
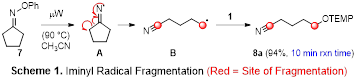
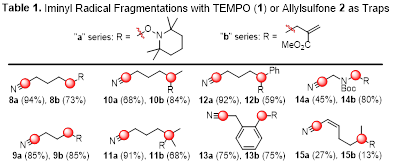
Our efforts in Year 2 of this PRF-funded project focused on pursuing Aim 2, which entailed exploring novel microwave-promoted fragmentations and g-C–H activations involving iminyl radicals. TEMPO (1, Figure 1) and compounds 2–6 were selected as radical traps to probe the ability to generate C–O, C–C, C–I, C–Cl, and C–N bonds using these reactions. We were pleased to find that microwave irradiation of a solution of oxime ether 7 and TEMPO in CH3CN afforded nitrile 8a in excellent yield in just 10 min, presumably via the intermediacy of cyclic iminyl radical A and acyclic alkyl radical B (Scheme 1). A variety of oxime ethers derived from 4- and 5-membered cyclic ketones underwent facile fragmentation under the same conditions in the presence of TEMPO or allylsulfone 2, furnishing nitriles 8–14 in good to excellent yields (Table 1). The lack of ring strain renders 6-membered cyclic oxime ethers less reactive, as an oxime ether derived from 6-methylcyclohex-2-en-1-one delivered nitriles 15a and 15b in low yields.
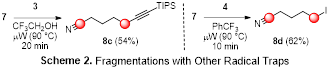
Encouragingly, fragmentation of 7 in the presence of benziodoxolone-based hypervalent iodine reagent 3 or i-PrI (4) produced adducts 8c and 8d in good yields after optimization of the solvent and reaction time (Scheme 2). Thus, we believe that the extension of this fragmentation method to include other radical traps is promising.
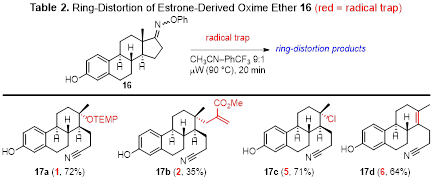
We then pursued fragmentations of estrone derived oxime ether 16 in order to show the utility of this method for generating diverse adducts from a complex natural product. This work was performed solely as a proof-of-concept study and not for the purpose of developing drugs or drug candidates. Fragmentation of 16 in the presence of TEMPO furnished ring-opened adduct 17a in good yield (Table 2). Fragmentations utilizing allylsulfone 2 were less facile, affording 17b in modest yield presumably due to the hindered nature of the new C–C bond. Carbon tetrachloride (5) was a viable trap, producing halide 17c in good yield. The use of sulfonyl azide 6 as the radical trap afforded alkene 17d as a result of HN3 elimination from the initial adduct.
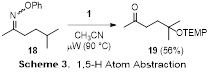
We briefly probed the ability of microwave-generated iminyl radicals to engage in 1,5-hydrogen atom abstractions. Irradiation of oxime ether 18 in the presence of TEMPO afforded ketone 19 in good yield after in situ hydrolysis of the NH imine intermediate (Scheme 3). Since the immediate precursor to 18 is a ketone, its two-step conversion into 19 constitutes a formal g-C–H activation. This is significant, as the g-position of a ketone is quite difficult to functionalize. We look forward to expanding on this preliminary result by examining other substrates and radical traps in the next year of the project.
Six students have worked on this project: MS student Mary Jackman, Ph.D. students Concordia Lo and Russell Denton, and undergraduates Siyeon Im, Seth Bohman, and Amanda Garrity. Mary has graduated and is currently teaching chemistry classes at Oakland University in Michigan. The PRF support she received was critical to facilitating her timely graduation. Siyeon will graduate in 2018, and her experience working on this project has inspired her to apply to Ph.D. programs in chemistry. Seth is planning on attending medical school upon graduation in 2018, but his research experience has been very positive and sparked an interest in performing research as a clinician. In addition to positively impacting these students, the PRF funding has been crucial to my own career. The results described above that we were able to obtain with PRF support have formed the basis of an NSF proposal that we submitted this week with the intent of securing funding for this project when the PRF award expires next year. Without PRF support, our preliminary results would have been much less impressive and our chances of receiving an NSF grant would be slim. Thus, I am very grateful that the ACS PRF was willing to fund a New Direction in my research at this time in my career.