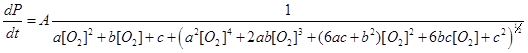Reports: UR456513-UR4: Synthesis of Alcohols, and Products Derived from Alcohols, from Hydrocarbons Using Only Solar and Simulated Solar Radiation
Patrick E. Hoggard, Santa Clara University
Background
To manufacture chemicals in the greenest manner possible, reactions should, when possible, be carried out at ambient temperature and pressure, using reagents that carry the smallest costs in energy. In particular, alcohols and aldehydes derived from hydrocarbons, which serve as primary reagents in countless synthetic chains, should, in the greenest of circumstances, be synthesized using sunlight as the energy source, molecular oxygen as the oxidizing agent, and a heterogeneous photocatalyst that can be easily recovered and recycled.
To this end, a number of laboratories have studied the photochemical oxidation of hydrocarbons with molecular oxygen, the vast majority using titanium dioxide as a catalyst. When irradiated, TiO2 is quite effective at abstracting hydrogen from hydrocarbons. The reaction of the alkyl radical with O2 proceeds and leads to a stable oxidation product. The disadvantages to TiO2 are that (a) there is effectively no light absorbed above 380 nm (TiO2 is white), thus only a small portion of the solar irradiance can be used; (b) TiO2 is so effective that oxidation does not stop at the aldehyde or ketone, and proceeds through the acid to CO2; (c) with TiO2 there is strong selectivity for the aldehyde or ketone as the initial product, which is undesirable if the alcohol is to be synthesized.
Our approach is to employ chlorine atoms to abstract hydrogen, using FeCl3 or FeCl4ˉ as the photoinitiator. Chlorine atoms are not as aggressive as the holes produced upon excitation of a TiO2 surface or the OH radicals that subsequently form. Iron-chlorine compounds absorb well into the visible and offer the possibility of harnessing a much greater fraction of the solar irradiance.
We began with cyclohexane. By far the main objective for the oxidation of cyclohexane is Nylon 6-6. Conventional oxidation methods produce a mixture of cyclohexanone and cyclohexanol, termed KA oil. This is used without separation to generate adipic acid, which is then reacted with hexamethylenediamine to make the polyamide.
Results
Experiments on the photooxidation of cyclohexane were initiated in the summer of 2017, with five undergraduate coworkers. A great deal of prior work on cyclohexane exists with TiO2 as the photocatalyst. Initially we compared photooxidation rates with several iron-chlorine catalysts: FeCl3 on silica gel, FeCl4ˉ on an anion exchange resin, and Et4N[FeCl4]. The last of these proved to be decisively the most effective. While soluble in most solvents, Et4N[FeCl4] is insoluble in cyclohexane.
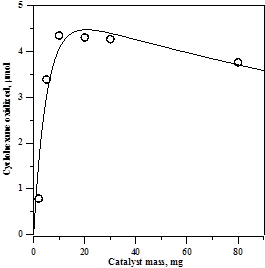
We have shown in the past that the rate of heterogeneously catalyzed photoreactions can pass through a maximum with respect to the amount of catalyst used, and this proved to be the case with Et4N[FeCl4], which yielded the highest rate using 10 to 20 mg/mL, as can be seen in the graph below. The solid line represents a fit to an equation derived earlier.1
Because O2 is both a reactant and a potential quencher of the Fe(III) excited state, we used a simple model to derive an equation to predict the variation in yield with the partial pressure of O2 over the reaction mixture. With parameters a, b, and c derived from rate constants, the equation takes the form
Using balloons to vary the fraction of O2 above the reaction, the product yield increased asymptotically towards a maximum at 100% O2, corresponding to the value of A/2c in the equation above.
Using a 100-W Hg lamp passed through a cutoff filter, the yield of cyclohexanol plus cyclohexanone surpassed that achieved by others with TiO2 as the catalyst. Furthermore, the reaction yield with a 395 nm cutoff filter was about one-third of the yield with a 320 nm cutoff, indicating that visible light makes a significant contribution to the yield. The photonic efficiency at 365 nm was found to be approximately 2%, under the specific set of conditions for that experiment, which is similar to what has been reported for TiO2 catalysts.
Unlike TiO2, the use of which leads to cyclohexanone to cyclohexanol ratios around 10 to 1, Et4N[FeCl4] led consistently to a ratio near 1 to 4, which could be useful were a photochemical synthesis of cyclohexanol to be undertaken. We have ascribed this shift by a factor of 40 in selectivity to the enhancement of one of the reaction channels,2 represented by the equation below, in which a cyclohexylperoxyl radical reacts with cyclohexane to produce cyclohexanol and a cyclohexoxyl radical, the enhancement caused by the isolation of the peroxyl radical after its formation in the solvent cage around the FeCl4ˉ site for a time sufficient to undergo reaction.
A number of experiments were undertaken in direct sunlight, without stirring the reaction mixture or using a lens to direct more light through the cyclohexane. Under these conditions approximately 0.5% conversion to oxidized products was achieved after six hours of irradiation. This underscores the difficulties to be faced in actually implementing photochemical methods to oxidize hydrocarbons, but there is still much room to optimize the conditions for such an operation.
(1) Hoggard, P. E.; Cohen, L. R.; Peña, L. A.; Harvey, B. M.; Chan, A. M. Curr. Catal. 2013, 2, 2.
(2) Hermans, I.; Nguyen, T. L.; Jacobs, P. A.; Peeters, J. ChemPhysChem 2005, 6, 637.