Reports: DNI1057062-DNI10: Ordered Mesoporous Metal Phosphides for Hydroprocessing
Xavier Roy, PhD, Columbia University
This ACS PRF program aims to develop a class of highly porous metal phosphide materials that could be deployed as heterogeneous catalyst for hydroprocessing of hydrocarbons. Current commercial catalysts for hydroprocessing based on transition metal sulfides are quickly reaching their efficiency limit as new, more stringent environmental regulations are enacted and the use of "dirtier" feedstocks becomes more prevalent.
Two strategies are under investigation: 1. Hard templating of mesoporous metal phosphide; and 2. Soft templating of mesoporous metal phosphide. We are initially targeting nickel phosphide in this project due to its demonstrated catalytic activity towards hydrodesulfurization and hydrodenitration. This report will primarily focus on our results concerning the first strategy; our progress with the second approach is very briefly discussed at the end of the document.
1. Hard templating of mesoporous metal phosphide.
The first step of this approach is to design soluble molecular precursors that can be converted to crystalline nickel phosphide at low temperature. We will infiltrate these precursors into a mesoporous template, and convert them to bulk crystalline phases. The template may then be removed to yield highly porous metal phosphide, or kept as part of the final material.
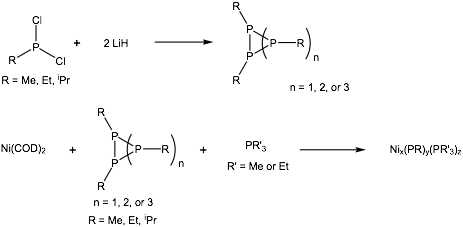
We have designed an entirely new family of nickel phosphide molecular clusters that can be converted to crystalline nickel phosphide upon thermolysis. Figure 1 presents the new synthetic strategy. Dichloroalkylphosphine, RPCl2, is first reacted with two equivalents of lithium hydride to form the organocyclophosphines (RP)n+2 via reductive dehalogenation and cyclization. The cyclic compounds are then reacted at room temperature in toluene with bis(1,5-cyclooctadiene) nickel(0), and an excess of trialkylphosphine (PR'3 with R' = Me, Et). Black crystals grow overnight from the reaction and are characterized by single crystal x-ray diffraction (SCXRD).
Figure 1. Synthesis of nickel phosphide clusters.
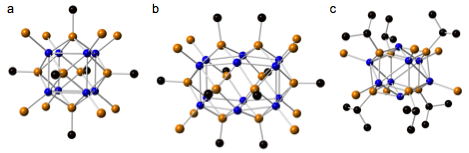
The size, composition and structure of the cluster can be tuned by changing the substituents R and R' on the organocyclophosphines and the capping ligand. Illustrating this point, Figure 2 presents the crystal structures of three nickel phosphide clusters, Ni8(PMe)6(PMe3)8, Ni12(PMe)10(PEt3)8 and Ni8(PiPr)6(PMe3)6, as determined by SCXRD. The core of Ni8(PMe)6(PMe3)8 is built from a cube of Ni atoms concentric with an octahedron of methylphosphinidenes (Figure 2a). Each methylphosphinidene caps a face of the cube, and is bonded to four Ni atoms at the corner of the cube, which are also capped with PMe3.
Figure 2. Crystal structures of (a) Ni8(PMe)6(PMe3)8 and (b) Ni12(PMe)10(PEt3)8 and (c) Ni8(PiPr)6(PMe3)6. The alkyl groups on the trialkylphosphine capping ligands, and the hydrogens are omitted for clarity. Legend: Blue, Ni; orange, P; black, C.
Using the slightly bigger capping ligand PEt3, instead of PMe3, yields the larger cluster Ni12(PMe)10(PEt3)8 (Figure 2b) The Ni12 core structure can be visualized as two face sharing Ni8 cubes, with each Ni4 face capped with one methylphosphinidene. The eight Ni atoms at the corner of the rectangular prism are capped with PEt3 while the four Ni atoms positioned at the belt of the prism are uncapped. Reacting (PiPr)3 with Ni(COD)2 and PMe3 gives a distorted PiPr-capped Ni8 cube, with six of the eight Ni atoms capped with PMe3 (Figure 2c).
The chemistry described above is new and the resulting clusters have never been reported. Other structures and compositions have been obtained using different combinations of capping ligands and organocyclophosphines.
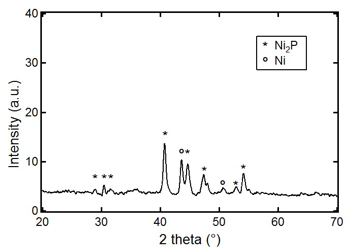
Importantly, these compounds bear no charge, are soluble in different organic solvents and their thermal decomposition leads to the formation of bulk nickel phosphide. Figure 3 illustrates this point for Ni12(PMe)10(PEt3)8. Thermolysis of Ni12(PMe)10(PEt3)8 in a sealed tube at 400 °C first results in the dissociation of PEt3, followed by the conversion of the core to crystalline Ni2P, as determined by powder x-ray diffraction (PXRD). These results are particularly exciting as it has been shown that such phases are highly active for the hydroprocessing reaction. Thermal decomposition studies of other nickel phosphide clusters are underway to determine whether new phases or compositions can be prepared.
Figure 3. PXRD pattern of the solid obtained via thermolysis of Ni12(PMe)10(PEt3)8.
In parallel to these studies, we are working on the fabrication of porous structures as templates for the formation of mesoporous nickel phosphides. We are initially targeting mesoporous silica, which we prepare using established protocols. The next steps in the coming funding period is to infiltrate this porous solid with a solution of the nickel phosphide cluster, and thermalize the resulting compound to convert it to a porous nickel phosphide-supported structure. Other hard templates, such as assemblies of monodisperse silica nanospheres, will be explored to create different morphologies. The atomic structure, mesostructure, porosity and activity of the mesoporous nickel phosphide materials will be characterized using crystallographic, microscopic and N2 adsorption isotherm techniques.
2. Soft templating of mesoporous metal phosphide.
We have developed a new amphiphilic nickel phosphide precursor whose headgroup is a Ni2+ cation, complexed with a long alkyl chain phosphine ligand to drive self-assembly in polar protic solvents. We have also demonstrated the ability of this precursor to react with sodium hypophosphite to form crystalline Ni2P. We are now beginning to study its liquid crystalline self-assembly into various solvents, including water, formamide and glycerol. The next step will be to use this amphiphilic coordination compound to template the formation of mesoporous nickel phosphide
During the first year, this ACS PRF grant has supported the efforts of one talented graduate student, Evan Doud, with one manuscript currently under preparation. In addition to supporting Evan, the Petroleum Research Fund has been crucial in broadening our research interests beyond traditional transition metal chalcogenide chemistry and into transition metal pnictide chemistry. This project has also allowed us to begin exploring materials design for catalytic applications, which I anticipate will become an important dimension of our work moving forward.