Reports: UNI155295-UNI1: Design, Synthesis, and Properties of Shape-Persistent Molecular Systems Comprising 1,3,5-Triethynylbenzene Cores
Derik K. Frantz, PhD, California Polytechnic State University
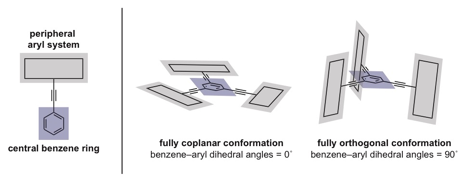
Over the past decade, arylethynylbenzenes have been applied toward the generation of carbon-rich molecules, liquid crystals, molecular knots, and materials with unusual optical and photophysical properties. Our efforts aim to explore the utility of the 1,3,5-triethynylbenzene moiety as a template for generating complex, shape-persistent structures in which the peripheral aryl systems are locked in coplanar and orthogonal conformations.
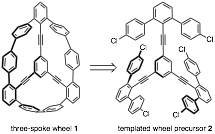
Our efforts toward a shape-persistent coplanar molecule have focused on three-spoke wheel 1. Over the past year, we have changed our original synthetic plan to feature a chloro-substituted templated wheel precursor 2. This molecule was envisioned as a direct synthetic precursor to 1 via Yamamoto homocoupling.
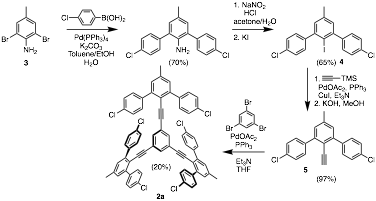
The trimethyl-substituted derivative (2a) of molecule 2 has been synthesized using a new strategy beginning with commercially available 2,6-dibromo-4-methylaniline (3). Suzuki coupling with 4-chlorophenylboronic acid, followed by a Sandmeyer reaction affords dichloroiodoterphenyl derivative 4. Subsequent Sonogashira coupling with TMS-acetylene and de-silylation yields alkyne 5, which is subjected to another Sonogashira coupling with 1,3,5-tribromobenzene to yield 2a. Yields for the final Sonogashira tri-coupling are currently sub-optimal, and the di-coupled, mono-bromo product is observed as the major product of the reaction when five molar equivalents of 5 are used in the coupling. Current work aims at increasing the yield of this reaction. It is worth noting, however, that the di-coupled product may be re-subjected to coupling conditions with another equivalent of 5 to yield 2a.
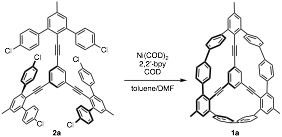
With a viable strategy to compound 2a in hand, our attention focused on the Yamamoto homocoupling to afford the trimethyl derivative of 1 (1a). We have found that slow addition of 2a to a dilute mixture of Ni(COD)2, bpy, and COD in DMF/toluene at 80 ˚C yields a complex mixture of products. The first fractions of a silica column contain 1a as determined by 1H NMR and high-resolution MS. Due to solubility issues, compound 1a has only been purified in 1–2 mg quantities. Current work is focused on improving the isolated yield of 1a and preparing the tri-n-hexyl derivative of 1 in order to increase its solubility.
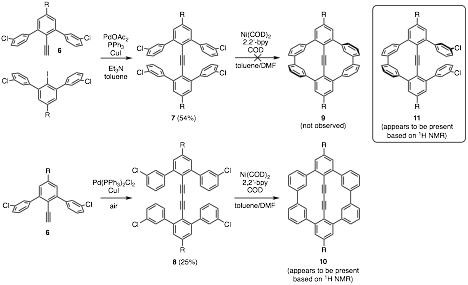
Comprising a central alkyne and peripheral chloride atoms, terphenyl 5 is a unique molecular construct. Based on the chemistry shown above, we have also developed a synthesis to the meta-chloro-substituted terphenyl derivative 6 with R = CH3 and n-hexyl. This molecular building block has been extended to the bis(terphenyl) alkyne 7 and diyne 8. Yamamoto couplings of these compounds could yield internally constrained derivatives (9 and 10) of [6]cyclo-meta-phenylene. These molecules will have unique and fascinating structural properties; molecule 9 is expected to exist as a twisted, D2-symmetric structure and molecule 10 is expected to adopt a C2v-symmetric structure whose alkynes bow out of the center of the macrocycle.
Yamamoto coupling of 7 has failed to yield 9, but appears to yield mono-coupled adduct 11. On the other hand, Yamamoto coupling of diyne 8 may have very recently yielded molecule 10. 1H NMR spectroscopy reveals the presence of a molecule whose chemical shifts match those that are expected from molecule 10. We cannot, however, conclusively state that these signals arise from compound 10. Current work aims at further characterizing this compound to determine its structure and optimizing reaction conditions.
The ACS-PRF award has been instrumental at the beginning of my independent career by supporting high-quality research opportunities for undergraduate students. The award has enabled a total of ten undergraduate students to take part in these projects and has directly supported four students in summer 2016 and four students in summer 2017. Major accomplishments, particularly the preliminary synthesis of 1a, were reached this year. The results will yield high-impact publications. We look forward to showcasing work supported by this award in publications and conferences in the coming year.