Reports: ND555043-ND5: Anion and Voltage Responsive Crystal Switching at Liquid-Graphite Interfaces for Ion Management in Non-Aqueous Solutions
Steven L. Tait, PhD, Indiana University
Amar H. Flood, PhD, Indiana University, Bloomington
Experimental methods, including scanning tunneling microscopy (STM), have greatly advanced our knowledge of self-assembly. In this year of ACS PRF funding, we have designed and synthesized a model molecular platform of alkoxybenzonitriles (ABN) to probe the competition between interactions in self-assembly at a surface. These molecules combine strong polar units (terminal nitriles) with van der Waals contact points (alkyl chains of varying length) to analyze the impact of each of these on assembly. This molecular system is relevant for studying 2D molecular self-assembly, as well as for future considerations of assembly and interactions with ions in solution. These experiments have built understanding of the behavior of the ABN molecular system at a range of concentrations for a variety of alkyl chain lengths.
Alkoxybenzonitriles are easily synthesized, can be intuitively designed with specific interactions, and serve as ideal specimens to progress understanding of self-assembly. This family of molecules is characterized by a very polar head, a benzonitrile group, bonded to through an ether to an alkyl chain. The alkyl chain length was modified to test the relative importance during self-assembly of dipole interactions at the benzonitrile head group vs. van der Waals interactions at the alkyl chain. These molecules were studied in octanoic acid as solvent, therefore columbic interactions with the solvent carboxylic head or vdW with the alkyl tail play a role in self-assembly.
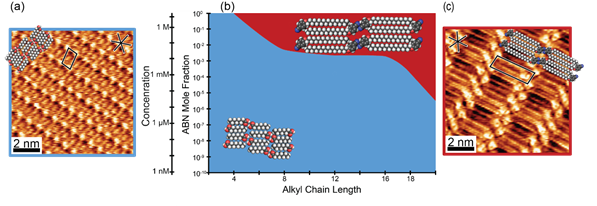
Alkoxybenzonitriles in octanoic acid is observed to self-assemble into a distinct packing phase. The structure of the phase is characterized using STM, after room temperature equilibration, to map the trends with chain length and concentration in a phase diagram (Figure 1). In these experiments, the concentration is varied from neat solvent to 8 M, and the alkyl chain length is varied from 4 to 18 carbons (C4 to C18). High concentrations produce a tightly packed alkoxybenzonitrile monolayer with interdigitated alkyl chains, while at lower concentrations only octanoic acid is absorbed and self-assemble. As the chain length of alkoxybenzonitriles is increased, the phase change from octanoic acid to alkoxybenzonitrile self-assembly occurs at lower concentrations.
Figure 1. High resolution STM images and packing models of alkoxybenzonitriles and octanoic acid at the solution/HOPG interface. (a) Octanoic acid self-assembly. Conditions: 1 mM ABN-C4, It= 0.23 nA, Vsample= -0.95 V. (b) Phase diagram of alkoxybenzonitriles in octanoic acid as function of alkyl chain length and concentration. Blue: octanoic acid self-assembly. Red: alkoxybenzonitrile self-assembly. (c) ABN-C18. Conditions: 10 mM, It= 0.40 nA, Vsample= -1.2 V.
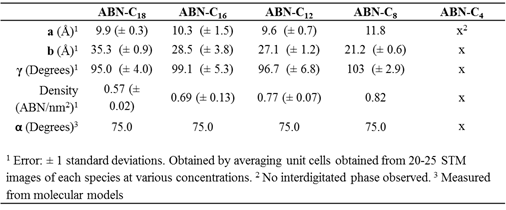
Table 1. Unit cell measurements and surface densities for alkoxybenzonitriles with different alkyl chain length.
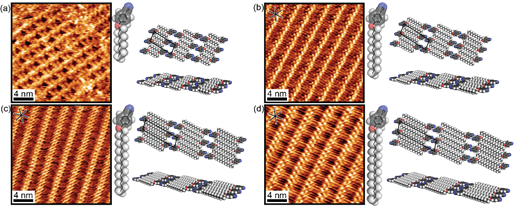
The molecular packing unit cell varies in an expected way with alkyl chain length, indicating that alkoxybenzonitriles are fully adsorbed (Figure 1 and Table 1). The most notable STM image feature of the self-assemblies is the distinct contrast between two types of rows (Figure 2). The double row of bright round features is attributed to head-to-head packing of the aromatic benzonitrile groups; aromatic groups usual appear in STM as higher contrast features than aliphatic groups. The lower contrast rows are assigned as the alkyl chains in an interdigitated packing.1-4 Images which resolve the HOPG surface with atomic resolution show that the alkyl chains are aligned with the zigzag lattice direction of the underlying HOPG. The alkyl-to-alkyl distance was measured to be 4.9 Å, which is slightly greater than the packing distance of 4.4 Å5-6 for pure alkanes on HOPG and thus produces a moiré pattern of varying contrast along the alkyl chain rows as the registry of the alkyl chains with the HOPG atomic rows oscillates.
Figure 2. STM images and molecular models of alkoxybenzonitrile interdigitation at the octanoic acid/HOPG interface with spacing filling models of respective molecules. (a) ABN-C8, Conditions: 8 M (neat ABN-C8), It= 4.5 nA, Vsample= -1.0 V. (b) ABN-C12, Conditions: 100 mM, It= 0.30 nA, Vsample= -1.1 V. (c) ABN-C16, Conditions: 100 mM,
It= 0.40 nA, Vsample= -1.0 V. (d) ABN-C18, Conditions: 10 mM, It= 0.40 nA,
Vsample= -1.2 V.
Octanoic acid self-assemblies are characterized by a single
row of bright features, and regular alternating contrast in lamellar rows (Figure 1). The
bright features are attributed to hydrogen bonding pairs of carboxylic acids
that from a resonant symmetric octagonal structure. From STM images, models are
constructed which features carboxylic acid heads forming hydrogen bonding dimers
with adjacent head groups, and alkyl chains interdigitating to produce lamellar
rows.
The boundary between alkoxybenzonitrile and octanoic
acid self-assembly is a function of alkyl chain length and concentration.
Larger molecular size in the form of increasing alkyl chain length increases
adsorbate-substrate and adsorbate-adsorbate vdW interactions. For example, ABN-C18
self-assembles at concentrations above 2 mM, but for ABN-C12,
concentrations above 50 mM are required for self-assembly.
1. Mali, K. S.; Feyter, S. D.,
Philos. Trans. R. Soc., A 2013, 371, no. 20120305.
2. Kudernac, T.; Lei, S.;
Elmans, J. A. A. W.; Feyter, S. D., Chem. Soc. Rev. 2009, 38,
402-421.
3. Mali, K. S.; Pearce, N.; De
Feyter, S.; Champness, N. R., Chem. Soc. Rev. 2017, 46,
2520-2542.
4. Teyssandier, J.; De Feyter,
S.; Mali, K. S., Chem. Commun. 2016, 52, 11465-11487.
5. Groszek, A. J., Proc.
Royal Soc. London A 1970, 314, 473-498.
6. Ilan, B.; Florio, G. M.; Hybertsen, M. S.; Berne, B. J.; Flynn,
G. W., Nano letters 2008, 8, 3160-3165.