Reports: DNI554840-DNI5: Understanding the Solid-Confined Ionic Liquid Nanofilms
Lei Li, PhD, University of Pittsburgh
Effect of Humidity on the Wettability of Ionic Liquids (ILs) on Mica Surface
1. Introduction
The wettability of ILs on the solid surface is crucial to many applications such as lubrication, catalysis and electrochemistry. Mica has been extensively used as the model solid in studying the IL/solid interfaces. It has been generally believed that electrostatic interaction is the key to the molecular structure of ILs on the mica surface. However, recent researches indicated that water adsorption is critical in determining the structure of ILs. If this is true, humidity should impact the wettability of ILs on mica dramatically.
2. Experimental
Three ILs, 1-butyl-3-methylimidazolium tris(pentafluoroethyl)trifluorophosphate (BMIM FAP), 1-butyl-3-methylimidazolium tetrafluoroborate (BMIM BF4) and 1-butyl-3-methylimidazolium bis(trifluoromethylsulfonyl)imide (BMIM NTf2), were acquired from EMD Millipore and used as received. Fresh mica surface is prepared by cleaving the muscovite mica sheets (McMaster Carr) with a pair of tweezers. Different humidity were obtained by using a series of saturated salt solutions in enclosed system. Specifically, they are NaOH (7% RH), CH3COOK (28% RH), NaI (48% RH), BaBr (60% RH), (NH4)2SO4 (80% RH) and K2SO4 (99% RH). Mica was put in the desired RH for at least 6 hours before contact angle measurements. The contact angles were measured with a VCA Optima XE using 2 mL drop sizes. ATR-FTIR measurements were conducted with a Bruker Vertec-70 LS FTIR and a Bruker Hyperion 2000 microscope in reflectance mode with Ge 20x ATR objectives. The spectrums were collected for 160 scans at 4 cm-1 resolution within the spectrum range of 1000cm-1 to 4000cm-1.
3. Results and Discussion
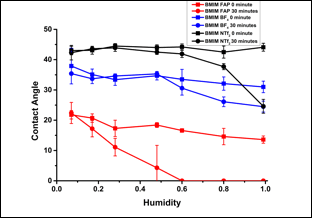
Figure 1 shows the contact angles of BMIM FAP, BMIM NTf2 and BMIM BF4 on mica under different RHs. Right after the IL droplets are placed on mica, the contact angles of BMIM FAP, BMIM NTf2 and BMIM BF4 under a RH of 7% are 22°, 43° and 38°, respectively. It is noteworthy that they were only slightly affected by the humidity increase. Thirty minutes later, the contact angles for all three ILs remain the same at low RH, i.e., 7%. However, the contact angles decrease with the increase of RH. This is especially prominent for BMIM FAP, which completely wet the mica surface, i.e., contact angle is 0°. BMIM BF4 and BMIM NTf2 shows the similar drop in the contact angle with the increase of RH though their final contact angles are higher than BMIM FAP.
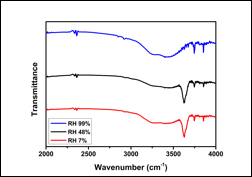
Previously, we proposed that the absorbed water enables the ion exchange between K+ on the mica surface and the cations of ILs since water is a much better solvent than ILs. When the cations of ILs occupy the empty site left by K+, the layering of ILs is initiated. If this is true, water adsorption should increase the wettability of ILs on mica and the results shown in Figure 1 do support this idea. To further confirm the mechanism, we conduct ATR-FTIR analysis to uncover the water adsorption on mica. As shown in Figure 2, at 7% and 48% RH, the broad peak located from 3200 cm-1 to 3500 cm-1 can be attributed to bulk water molecules and the sharp peak at 3600 cm-1 can be attributed to the bonded water on mica. Interestingly, only one broad peak with detected when RH is 99%, indicating large amount of water adsorption so that bulk water dominates the spectrum. These findings supports the hypothesis that water adsorption is the key to the enhanced wettability.
4. Summary
We have measured contact angles of three ILs on mica under different humidity and characterized water adsorption on mica with ATR-FTIR. The results indicate that wettability of ILs on mica increase with humidity, which can be attributed to the enhanced layering of ILs induced by the water adsorption.
Figure 1. Contact angle of ILs on mica under different RH. ("0 minute" is the contact angle taken right after the IL drop is placed on mica. "30 minute" is the contact angle taken 30 minutes after the IL drop is placed on mica.)
Figure 2. ATR-FTIR spectrum of a fresh mica surface under various RH.