Reports: ND155108-ND1: Intermolecular Aliphatic C-H Functionalization Using Heteroatom-Centered Radicals
Erik J. Alexanian, University of North Carolina, Chapel Hill
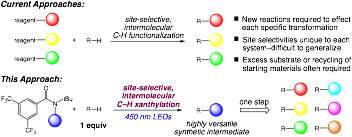
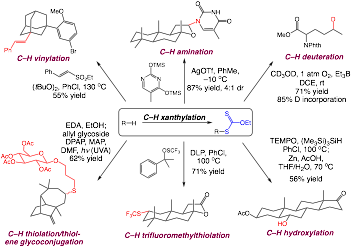
Synthetic methods that selectively functionalize unactivated aliphatic C–H bonds hold significant promise in streamlining the synthesis of complex targets and offer attractive tools for late-stage functionalization. We have previously developed an approach to the selective halogenation of unactivated C–H bonds using N-haloamides and nitrogen-centered radicals. During the past grant period, we have demonstrated the versatility of a platform for C–H functionalization using N-xanthylamides. This approach capitalizes on the established polar and radical chemistry of alkyl xanthates to access a wide array of functional groups via a new, diversity-oriented approach to C–H functionalization.
The aliphatic C–H xanthylation unlocks an array of important C–H transformations, include many that have no synthetic precedent. This C–H functionalization also demonstrates good functional group compatibility and useful site selectivities across a range of complex substrates. We anticipate that the scope and practicality of this C–H functionalization platform will lead to diverse applications in molecular derivatization in a number of contexts.
We thank the ACS PRF for their generous support of this work, which has played an instrumental role in our efforts towards the development of practical, selective methods for intermolecular aliphatic C–H functionalization.