Reports: DNI155070-DNI1: Light-Driven Dehydrogenative Carbon-Carbon Coupling Reactions
Christopher Uyeda, Purdue University
The overarching goal of our ACS PRF-supported research program is to study the applications of heterogeneous semiconducting materials as photoredox catalysts for organic transformations. Photoredox catalysis has recently emerged as a powerful approach for promoting challenging transformations by exploiting low-barrier single electron reaction pathways. A majority of current methods are reliant on molecular photosensitizers, which function as outer-sphere electron transfer reagents in their excited states. In principle, heterogeneous materials offer several attractive features as potential catalysts. (1) Their insolubility facilitates catalyst recycling and applications in flow systems were immobilization is desirable. (2) The band gaps of heterogeneous catalysts can be tuned through nanostructuring. For example, quantum dots exhibit size-dependent absorption spectra that would allow access to a continuous range of excited state redox potentials. By contrast, homogeneous photosensitizers must be tuned through synthesis and are limited by the accessibility and stability of various substitutions. (3) Substrate–surface interactions may provide an avenue to obtain unique selectivities that are not accessible in mechanisms that are governed strictly by outer-sphere electron transfer.
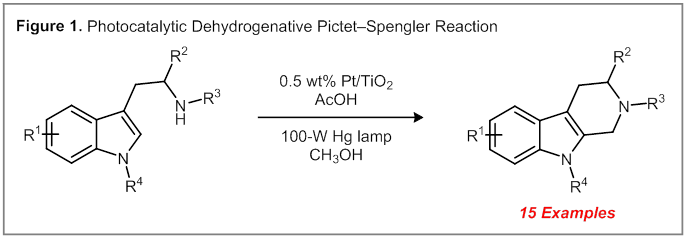
Dehydrogenative Pictet–Spengler Cyclization. Pt/TiO2 catalysts are known to promote the dehydrogenation of alcohols under visible light illumination. While this reaction has been demonstrated in simple transformations, the compatibility of this catalyst system with more complex and densely functionalized substrates had yet to be established prior to our work. We initiated our studies by examining the ability of Pt/TiO2-catalyzed alcohol dehydrogenations to be conducted in tandem with a Pictet–Spengler cyclization reaction. This reaction was selected as a model due to the known sensitivity of electron-rich heterocycles and aliphatic amines to oxidative conditions. Under optimized conditions, a range of tetrahydro-β-carboline products were obtained in high yield (Figure 1).
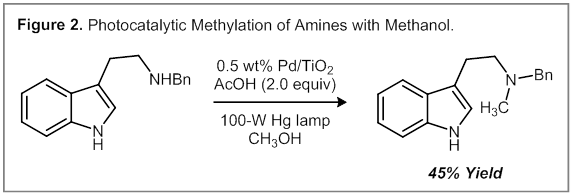
Redox-Neutral Alkylation Reactions. A minor side product that was observed in the Pictet–Spengler reaction is an N-alkylation product of the primary amine (6% yield). We reasoned that this competing process may involve an addition of H2 to the putative iminium ion intermediate. Overall, this reaction constitutes a redox-neutral alkylation of an amine with an alcohol that proceeds through a dehydrogenation–hydrogenation sequence. We have been exploring alternative catalysts that may provide improved yields in this reaction. Accordingly, a Pd/TiO2 catalyst was found to produce up to 45% of the N-methylated product, presumably due to the superior hydrogenation activity of Pd vs. Pt. Based on this initial result, we are currently optimizing a set of conditions for the alkylation of heteroatom functional groups using alcohols.
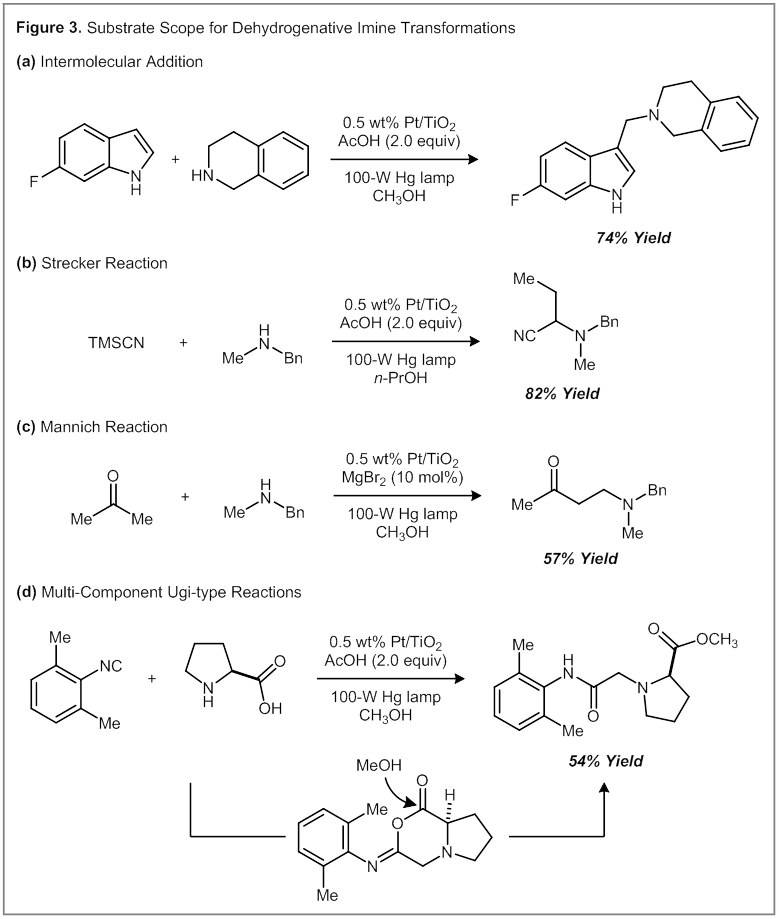
Dehydrogenative Variants of Other Imine-Based Transformations. Using the conditions optimized for the Pictet–Spengler reaction, we have extended the scope of this photoredox dehydrogenation strategy to other reactions of imines, including intermolecular additions of indoles, Strecker reactions, Mannich reactions, and multi-component Ugi-type reactions (Figure 3). Additionally, we have applied these conditions to a one-step synthesis of Lidocaine. As part of these experiments, we uncovered an interesting tandem Ugi reaction coupled with a methyl ester formation. A proposed mechanism is illustrated in Figure 3, and involves an attack of the pendant carboxylic acid on a nitrilium intermediate. Subsequent ring-opening with MeOH then generates the methyl ester product.
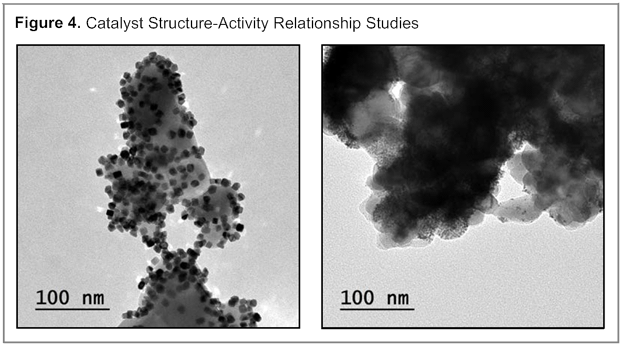
Catalyst Structure–Activity Relationships. A long-term goal of this project is to use the tools of materials synthesis order to tune the catalytic activity of heterogeneous catalysts for the dehydrogenation reaction. Toward this goal, we have initiated a collaboration with Prof. Tykhon Zybkov at Ball State University. A series of new catalysts were prepared using thermal and photodeposition techniques, leading to variations in the size and morphology of the deposited Pt (Figure 4). In our initial studies, we have observed that these factors have a significant impact on the rate of the dehydrogenative Pictet–Spengler reaction and the selectivity for cyclization vs. N-methylation. Ongoing efforts are directed at conducting systematic structure–activity studies.
Impact. Over the past grant year, we have completed our studies on the Pictet–Spengler reaction (15 examples) and significantly extended the scope of viable imine-based transformations. Additionally, we have conducted an initial study on redox-neutral alkylation reactions. Overall, we view this work as providing a proof-of-principle that heterogeneous semiconducting materials, commonly used in solar energy conversion applications, may exhibit attractive catalytic properties in organic transformations.
Two graduate students who collaborated on this work were directly funded by this PRF grant. These students had extensive experience in the techniques of organic synthesis and were exposed through this project to concepts in materials science, including the preparation and catalytic properties of metal oxide photocatalysts. Additionally, we established a collaboration with Tykhon Zubkov, who conducted research with undergraduate students on the synthesis of nanoparticle catalysts.