Reports: UNI354419-UNI3: Mechanistic Investigations of Aryl-SO2-Heteroatom Bond Formation from Palladium Using Bench-Stable SO2 Sources
Nicholas D. Ball, PhD, Pomona College
Project Narrative 2017
During the 2016-2017 grant year, I was able to support two students with my ACS PRF grant: Bryan Santti and Sabrina Kwan. This narrative will detail the progress made on the specific aims of the funded research. Recent efforts have focused on Pd and Cu-catalyzed methods towards the generation of sulfonylated compounds using bench-stable SO2 sources DABSO (1,4-Diazabicyclo[2.2.2]octanebis(sulfur dioxide)) sodium sulfite, or potassium metabisulfite. These solid SO2 reagents eliminate the need for the synthesis and isolation of sulfonyl chlorides, and represent a considerable advance due to their ease of handling, minimal inhalation hazard, and commercial availability. To move this field forward, my research group has focused on using these transition metal-based catalytic platforms to synthesize sulfonyl fluorides as well as develop versatile methods to activate sulfonyl fluorides as starting materials towards an array of sulfonylated molecules.
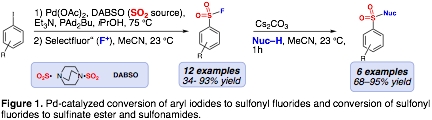 This year, we published work in this field focused on Pd-catalyzed generation of aromatic sulfonyl fluorides from aryl iodides using DABSO and Selectfluor as SO2 and fluoride sources, respectively. Additionally, we demonstrated by using Cs2CO3, sulfonyl fluorides can be activated to react with aromatic alcohols and amines to generate sulfonic esters and sulfonamides; however, analogous reactions with benzylic or aliphatic alcohols and amines were not successful (Figure 1).
This year, we published work in this field focused on Pd-catalyzed generation of aromatic sulfonyl fluorides from aryl iodides using DABSO and Selectfluor as SO2 and fluoride sources, respectively. Additionally, we demonstrated by using Cs2CO3, sulfonyl fluorides can be activated to react with aromatic alcohols and amines to generate sulfonic esters and sulfonamides; however, analogous reactions with benzylic or aliphatic alcohols and amines were not successful (Figure 1).
Computational and mechanistic work are on-going towards novel SO2 insertion into Ni and Pt halide complexes and subsequent formation of R-SO2-X bonds (where R is a carbon group and X is a halogen). In addition, we are investigating intercepting these SO2 insertion products with carbon and nitrogen coupling partners leading to mechanistic precedence toward Ni and Pt catalyzed functionalization of organic molecules to sulfones and sulfonamides.
Future Directions
Ongoing work is focused on new methods to activate sulfonyl fluorides towards other sulfonylated groups including sulfonamides. Computational and mechanistic work are on-going toward novel SO2 insertion in Ni and Pt heteroatoms bonds.











