Reports: ND356558-ND3: Models for Heterogeneous Single Atom and Small Cluster Catalysts on Graphene: A Novel Gas Phase Approach
Scott Gronert, Virginia Commonwealth University
Introduction
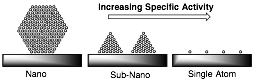
In this grant, we are using a novel gas-phase approach to study simple models of heterogeneous catalysts based on single metal atoms supported on aromatic platforms. These systems offer the potential for very efficient catalysis and are a natural progression from nano-based systems (Figure 1)
Figure 1. Effect of catalyst size on specific activity.
Specifically, neutral metal atoms supported on charged polycyclic aromatics are being probed in the gas-phase with an ion trap reactor. Our working hypothesis is that the studies will allow us to examine individual steps in catalytic cycles, survey the reactivity of a broad set of metals and small clusters supported on model polycyclic aromatic surfaces, and develop deep insights into the chemistry of the supported metal atoms. Our rationale is that by carefully controlling the conditions and isolating specific complexes and intermediates, we will be able to make links between reactants and products, and the factors that control reactivity, that would be very difficult to accomplish in the mix of species and surfaces found in condensed-phase systems. The specific aims are:
Specific Aim #1. Develop and optimize synthetic methods to generate metal atoms supported on polycyclic aromatic ions. Our working hypothesis is that oxalate decarboxylation or related approaches will offer pathways to neural metal atoms on aromatic platforms.
Specific Aim #2. Characterize the reaction processes of metal atoms supported on polycyclic aromatic ions. Our working hypothesis is that the complexes will act as suitable models for single atom catalysts and the metals will engage in fundamental processes such as oxidations (via molecular oxygen activation) and oxidative additions.
Results
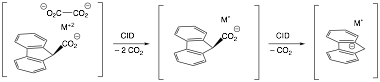
In our initial studies, we have demonstrated that it was possible to generate cobalt atoms on a flourenyl anion support via sequential decarboxylation reactions. The process begins with a two-electron reduction of Co(II) by the fragmentation of an oxalate dianion and is followed by the decarboxlylation of the 9-fluorene carboxylate ligand (Figure 2). We have extended these results along paths related to Specific Aims 1 and 2. First, we have established that the methodology is also viable with Ni(II) and a similar complex can be formed via the same methodology. Second, we have established that the Co and Ni fluorenyl complexes are capable of 1- and 2-electron reductions of alkyl and vinyl halide substrates. With heptafluoroiodopropanes, one electron reductions leading to M(I)-I complexes are observed. These products are prone to doing a second 1-electron reduction to produce M(II) di-iodides. With trichloroethene, 2-electron reductions to give M(II)Cl2 complexes with the loss of an alkyne are observed. This pathway is likely the result of oxidative addition followed by a breakdown of the metal-halovinyl complex. In each case, the reactivity is consistent with expectations for a supported, single metal atom catalyst.
Figure 2. Synthesis of Mo complex of flourenyl anion.
Challenges/Changes in Reaction Plan
The research plan has worked to date and we do not plan any significant changes. The project suffered a personnel challenge when the graduate student funded by the grant went on personal leave followed eventually by resignation from the graduate program. The uncertainty in this months-long process delayed reassigning a new student to the project and led to a major delay in the first year. With a dedicated PhD student now in place, the project is moving forward at a solid pace. We have recruited an undergraduate for the project and plan to add a second in the spring. In another change, the PI has accepted a 50% role in the Dean's office on an 11-month contract (Associate Dean of Research), so no summer salary funds will be drawn from the grant and those funds will be redirected to student support.
Next Steps
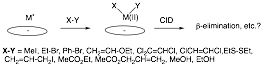
Having established viable syntheses of the cobalt and nickel systems, we will move to the study of reaction chemistry. The survey systems are shown in Scheme 1. We expect a broad range of reactivity with these substrates. These studies are being led by a PhD student, but an undergraduate is being recruited to help accelerate them.
Scheme 1. Systems for surveying reactivity of Mo systems.
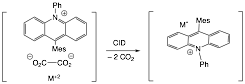
One of the goals of the project is to contrast anionic with cationic aromatic platforms. An undergraduate has been tasked with developing a similar synthetic pathway based on an acridinium platform. Once formed, these complexes will be treated with the same reagents as in Scheme 1.
Scheme 2. Acridinium platform for Mo systems.
In the final phase for the next year, a graduate student will reconfigure our neutral reagent system for handling gaseous reagents (we currently inject liquids via a syringe pump). We have had a similar system in the past. This will allow us to study the chemistry of the Mo complexes with H2 and CO/O2 mixtures. These reactions will produce intermediates most relevant to synthetic chemistry applications, with the former involved in hydrogenations of alkenes and alkynes, and the latter with the oxidation of CO to CO2.
Summary
The first year included a personnel challenge that has been resolved, but delayed progress by several months. The chemistry however is going well and we have expanded the synthetic scope of the Mo production pathway and identified typical Mo processes with the resulting complexes. With a PhD student dedicated to the project and a plan to have two undergraduates, we anticipate a very productive year two for the project.