Reports: DNI556712-DNI5: Selective Hydrogenolysis of C-Cl Bonds: A Fundamental Reaction Kinetics Study
Thomas J. Schwartz, PhD, University of Maine
Impact of the Proposed Research
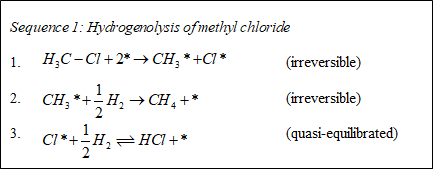
The selective hydrogenolysis of carbon-X bonds, where X can be a carbon, oxygen, nitrogen, sulfur, or a halide such as chlorine, is an important class of reactions in the production of chemicals from petroleum feedstocks. In particular, the synthesis of certain chlorobenzenes and chlorophenols from benzene and phenol, respectively, involves the selective hydrodechlorination of polychlorinated species.1-2 This reaction is attractive not only because it provides a use for unwanted byproducts (i.e., over-chlorinated benzenes or phenols) but also because it can provide access to certain isomers not obtainable by other means (e.g., species chlorinated in the meta position). While much of the research in the petrochemical field has historically focused on the cleavage of C-C and C-N bonds, the cleavage of C-Cl bonds shares many features with these better-studied reactions. According to Sinfelt, the hydrogenolysis of ethane, methylamine, and methyl chloride all proceed by a similar mechanism, shown in Sequence 1,3 and in all three cases, the rate-controlling step in the reaction is the cleavage of the C-X bond.
Inspired by the lack of fundamental, molecular-level insights into the hydrogenolysis of carbon-chlorine bonds in aromatic systems, this project aims to study this reaction in detail. Such understanding is imperative for the design of new catalytic materials. Our research objectives focus on elucidating the reaction kinetics and mechanism for C-Cl hydrogenolysis using two common catalysts: Pd and Ni. Discrepancies in the reaction kinetics data presented in the literature suggest that this reaction may occur by a different mechanism over each catalytic material; although such a difference would be in contrast to the observations of Sinfelt for hydrogenolysis reactions in general.4 We hypothesize that the hydrogenolysis mechanism should actually be equivalent for both Pd and Ni, and we are undertaking to test this hypothesis by combining classical reaction kinetics measurements, isotopically labeled experiments, and in situ Fourier transform infrared (FTIR) studies.
Impact of the ACS-PRF Award on Students and the PI
This award has supported the research activities of two PhD students (Mohammed Al-Gharrawi and Jalal Tavana) and one undergraduate (Aiden Crane). Mohammed has focused on the construction and operation of a gas-phase mixed-flow reactor used to directly measure reaction rates, while Jalal has focused on the construction and operation of an in situ FTIR apparatus for observing the surface of working catalysts under reaction conditions. Both students attended the spring symposium of the New England Catalysis Society in 2017. Additionally, Mohammed presented his preliminary data at the UMaine Student Symposium on Research and Creative Activity in 2017. All three students have learned to perform a variety of experimental techniques, including measuring reaction kinetics in both gas-phase and liquid-phase systems, gas chromatography and high-performance liquid chromatography, volumetric chemisorption measurements, and FTIR spectroscopy.
This award has had a significant impact on the PI, with funding from ACS PRF helping support a new research direction focused on C-X hydrogenolysis reactions in various systems. The ideas explored in this work, coupled with similar observations in other projects currently underway, will form basis for future proposals and a long-term research focus in C-X bond cleavage reactions. Moreover, the work from this project will result in several publications and has already facilitated the development of significant research infrastructure in our laboratory.
Preliminary Results
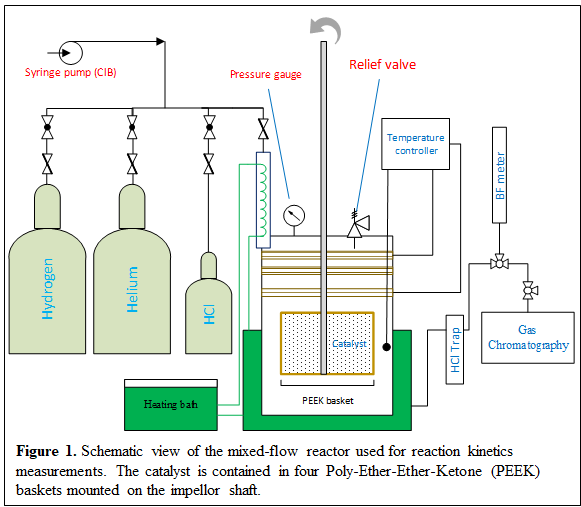
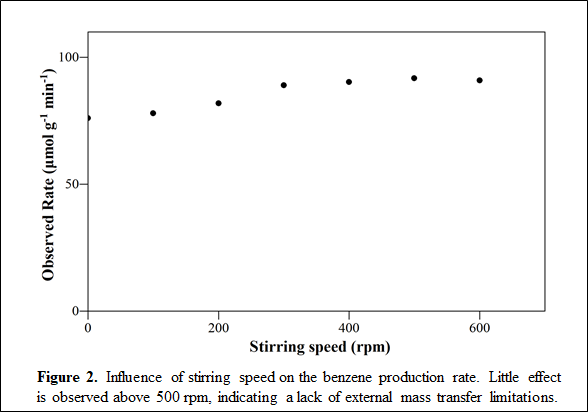
Construction of Mixed-Flow Reactor: A new reactor system was constructed to carry out these studies, shown in Figure 1. A glass body reactor was chosen to avoid corrosion from the HCl produced during the reaction. A mixed-flow reactor was chosen to facilitate the direct measurement of reaction rates even at high conversion, with the catalyst contained in a home-made Carberry impellor. As shown in Figure 2, this configuration operates free of external mass transport limitations.
Construction of in situ FTIR Cells: Construction is ongoing on a pair of in situ transmission FTIR cells and an accompanying volumetric dosing system. One cell is designed for collecting spectra of probe molecules adsorbed on pre-treated catalyst samples. Coupled with a calibrated volumetric dosing system, this cell will be used for the measurement of molar extinction coefficients. The second cell is configured to observe working catalysts under reaction conditions.
Preliminary Reaction Kinetics Results: The reaction kinetics of chlorobenzene hydrogenolysis have been measured over a low-dispersion Pd/C catalyst. This system shows no deactivation over 50 hours of time-on-stream, consistent with the observations of Aramendia and coworkers.5 Thus, catalyst deactivation in this system appears to be structure sensitive; on-going work in our lab will verify this conclusion.
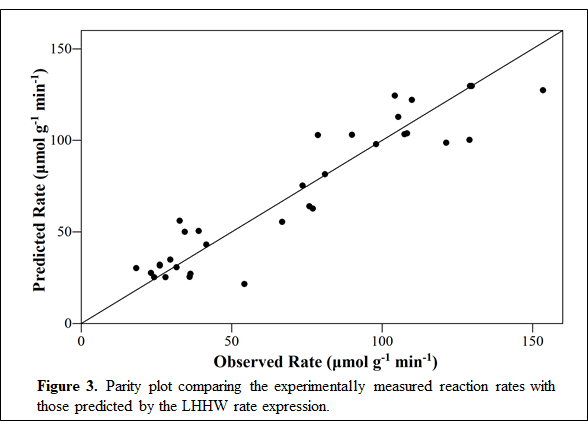
The production rate of benzene over this catalyst depends in a complex fashion on the partial pressure of chlorobenzene: the reaction shifts from positive-order at 353 K to near-zero-order at 313 K. A similar effect was observed for this reaction with respect to hydrogen pressure. Both observations suggest a mechanism that is consistent with Sinfelt’s description (see Sequence 1). Cleavage of the C-Cl bond in chlorobenzene is rate-controlling and irreversible while hydrogenation of Cl* and desorption of HCl are quasi-equilibrated. Notably, while chloroalkanes must undergo dissociative chemisorption, the aromatic ring in chlorobenzene can itself bind to the catalyst surface, which gives rise to the observed shift in reaction order at low temperatures. The kinetics data can be well-described by a Langmuir-Hinshelwood-Hougen-Watson rate expression corresponding to this mechanism, as shown in Figure 3.
References
1. Krishnamurti, R., Chlorinated Benzenes. In Kirk-Othmer Encyclopedia of Chemical Technology, John Wiley & Sons, Inc.: 2000.
2. Desmurs, J.-R.; Ratton, S., Chlorophenols. In Kirk-Othmer Encyclopedia of Chemical Technology, John Wiley & Sons, Inc.: 2000.
3. Sinfelt, J. H., Catalytic hydrogenolysis on metals. Catalysis Letters, 1991, Volume 9, 159-171.
4. Sinfelt, J. H., Specificity in Catalytic Hydrogenolysis by Metals. In Advances in Catalysis, D.D. Eley, H. P.; Paul, B. W., Eds. Academic Press: 1973; Vol. 23, pp 91-119.
5. Aramendia, M. A.; Borau, V.; Garcia, I. M.; Jimenez, C.; Lafont, F.; Marinas, A.; Marinas, J. M.; Urbano, F. J., Influence of the Reaction Conditions and Catalytic Properties on the Liquid-Phase Hydrodechlorination of Chlorobenzene over Palladium-Supported Catalysts: Activity and Deactivation. Journal of Catalysis, 1999, Volume 187, 392-399.