Reports: ND756632-ND7: Multi-Scale Modeling of Dynamics of Polymer Gels in Oil-Water Mixtures
Olga Kuksenok, Clemson University
Polymer gel treatments play an important role in enhanced oil recovery (EOR) techniques1. We focus on gel dynamics on multiple length scales: the Dissipative Particles Dynamics (DPD)2 formulation allows us to capture the specifics of the interaction of the gel particle with the oil-water interface at nanoscale, while a three dimensional gel Lattice Spring approach (gLSM)3 focuses on mm-scale samples. In the latter case, we account for temperature-dependent cross-links4 that could potentially be used in the deep reservoir treatments1, 10.
Polyacrylamide (PAM) are known to increase water viscosity and oil sweep ability.6 The preformed particle gels (PPGs) are used to control conformance and increase the sweep efficiency by diverting the fluid to unswept oil zones. PAM PPGs can be prepared by solution polymerization or by inverse suspension/emulsion polymerization.7 Another method of synthesis of PAM involves polymerization of acrylamide with poly(ethylene glycol) diacrylate (PEGDA) using N,N’-methylene-bisacrylamide (MBAM) as crosslinkers4. MBAM is a thermally stable crosslinker, while PEGDA crosslinker undergoes cleavage4 at 80°C; thereby, these gels can effectively seal pores with matched pore size.
DPD simulations of polymer
gels at oil-water interfaces. We developed a DPD framework to simulate
a PAM gel network immersed into the water-oil mixtures. We designed the gel
matrix as a diamond cubic lattice8 where the lattice points are occupied
by tetrafunctional crosslinks (MBAM) connected by the acrylamide polymer chain (Figure
1a). Nx represents the number of polymer beads between the
crosslinks. Simulations were performed using LAMMPS simulation package.
Interaction parameters were set at9 aij=78 for the same
type of beads and at aij=100 for the oil-water interactions.
Periodic boundary conditions were applied in all directions.
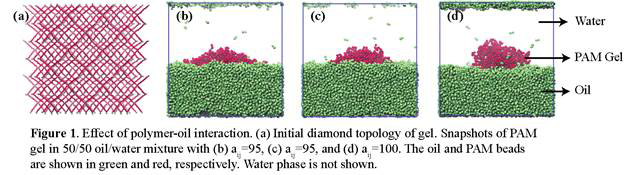
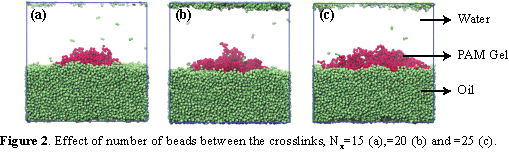
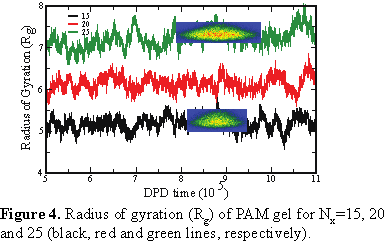
First we focused on the PAM gel immersed in 50/50 oil-water mixture and varied polymer-oil interaction parameter: aij=90, 95, and 100, respectively in Figure 1b-d. Our results show that the low values of aij promote spreading of the nanogel over the interface We then varied Nx,, setting it to 15, 20 and 25, respectively (Fig. 2 a-c), while keeping aij=95. Effective spreading of the gel is observed for low volume fraction of crosslinks (Nx=25). Density plots in Figure 3 confirm that the increase in Nx promotes gel spreading; furthermore, an increase in Nx results in a systematic increase in the radius of gyration of the nanogel, Rg (see Figure 4). The developed framework allows us to tailor interactions between the gel particle and oil-water interfaces depending on crosslink density and polymer-oil interactions.
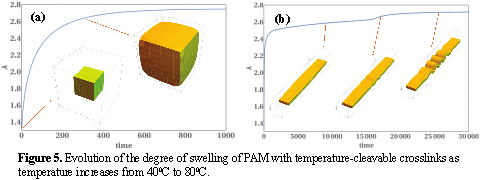
gLSM simulations of gels with temperature-sensitive cross-links One of the challenges in deep reservoir treatments is reducing the permeability of the thief zone only when this treatment reaches deep regions; this issue could potentially be addressed by using dual crosslinked gels with thermally breakable crosslinks1, 10. We adapt gLSM3 approach to model PAM gel particles with thermally cleavable crosslinks. Fig. 5 shows the evolution of the degree of swelling of gels with thermally cleavable crosslinks when temperature is increased from 400C to 800C. In Fig. 5a, the initial linear dimension of a cubic sample is 1 mm at 400C; the degree of swelling increases by a factor of 2.75 at 800C. This value is matched with the parameters from experimental data from ref 4, while interaction parameter for PAM is taken from ref 11. Fig. 5b shows pattern formation and propagation in a thin film of the same gel (initial length is 8mm).
Increasing thermal stability of enzymes. Another direction in current EOR approaches is enzyme EOR or (EEOR)5. High temperatures in deep wells makes thermal stability of the enzymes essential for these applications.5 Together with concurrent experimental studies12, we focused on a novel conjugation approach that has a potential to be applicable to a range of enzymes. Our results show that conjugation of a sample enzyme (lysozyme) with a tailored copolymer results in a significant increase in the structural stability12 of this enzyme at temperatures above 1200C.
Portions of this work were presented at the invited lecture at a Gordon Research Conference, Ventura, CA (Jan 29 - Feb 3, 2017) and at the Keynote Lecture, ChEmference 2016, IIT Gandhinagar, India; one manuscript is under review and two manuscripts are in preparation.
1. Muggeridge, A., et al., Philos T R Soc A 2013, 372 (2006).
2. Groot, R. D., et al., The Journal of Chemical Physics 1997, 107 (11), 4423.
3. Kuksenok, O., et al., Physical Review E 2008, 78 (4), 041406.1.
4. Chen, Z., et al., Journal of Applied Polymer Science 2017, 134 (13), 44581.
5. Patel, J., et al., Renewable and Sustainable Energy Reviews 2015, 52, 1539.
6. Yegin, C., et al., Society of Petroleum Engineers: Mumbai, India, 2017.
7. Li, G., et al., Energy & Fuels 2013, 27 (11), 6632.
8. Yong, X., et al., Nano Letters 2013, 13 (12), 6269.
9. Nair, N., et al., The Journal of Physical Chemistry B 2016, 120 (35), 9523.
10. Pritchett, J., et al., Society of Petroleum Engineers: 2003
11. Kizilay, M. Y., et al., Macromolecules 2003, 36 (18), 6856.
12. Yadavalli, N. S., et al., ACS Catalysis 2017, Submitted.