Reports: ND756819-ND7: Investigating Polymer-Metal Bistriflimide Interactions for Functional Materials Design
Jason E. Bara, PhD, University of Alabama, Tuscaloosa
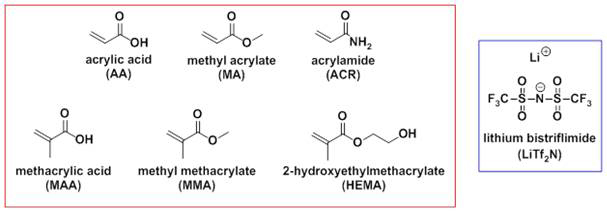
We have successfully studied the polymerization behaviors of a number of “coordinated” ionic liquid (IL) systems thus far. As outlined in the original proposal, we first studied the photopolymerization behavior of several acrylate- and acrylamide-based monomers in the presence of lithium bistriflimide (LiTf2N) as depicted in Figure 1.
Figure 1: Structures of acrylate- and acrylamide-based monomers studied in combination with LiTf2N.
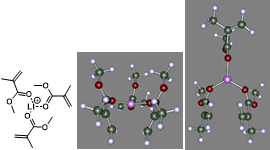
Using both FT-IR data and the COSMOTherm software, we demonstrated that the Li+ cation coordinates to the carbonyl moiety in these species, with a 3:1 ligand:Li+ ratio most preferred. Figure 2 illustrates the arrangement of 3 MMA ligands about a Li+ cation. The Tf2N- anion is essentially a spectator as it is non-coordinating toward the Li+ cation in the presence of more strongly binding species/functional groups.
Figure 2: Coordination of Li+ by 3 MMA ligands. Left: 2-D structure. Center and right: two views of the optimized 3-D “ball and stick” structure generated in COSMOTherm.
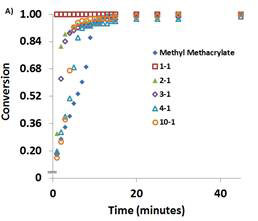
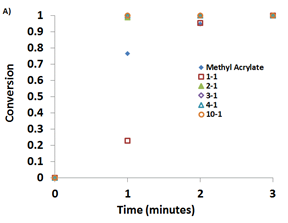
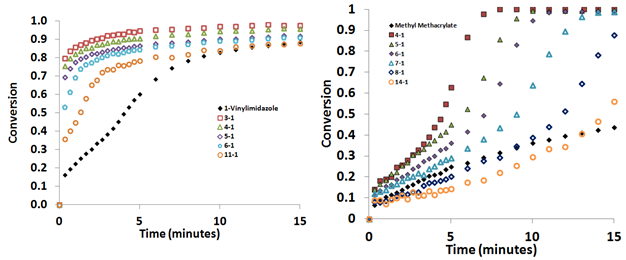
Photopolymerization experiments were performed using the six monomers shown in Figure 1 with at various monomer:LiTf2N ratios (e.g. 1:1, 2:1, 3:1, 4:1, 10:1) so as to study the effect of the monomer:LiTf2N ratio on the photopolymerization rate and monomer conversion. Results for MA and MMA are shown in Figure 3.
Figure 3: Comparison of monomer conversion with time for MMA (left) and MA (right) systems with various ratios of monomer:LiTf2N.
Figure 3 illustrates that the presence of LiTf2N creates a much greater difference in the rate of monomer conversion in MMA than in MA. For MMA, the 1:1 MMA:LiTf2N system fully polymerizes in less than 1 min, while increasing the MMA:LiTf2N ratio resulted in slower conversion of monomer to polymer, with the neat MMA exhibiting much slower rate than any system which contained LiTf2N. For MA, the presence of LiTf2N made little difference in the behavior as all MA:LiTf2N ratios achieved ~100% monomer conversion within 2-3 min. Studies on the other 4 monomers also confirmed that adding LiTf2N to these monomers promoted enhancements in overall monomer conversion and rate.
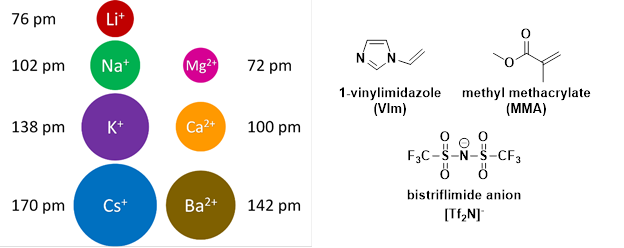
Using MMA and 1-vinylimidazole, similar studies were performed using alkali and alkaline earth metal bistriflimide salts (Mn+(Tf2N-)n) as illustrated in Figure 4.
Figure 4: Left: Diagram displaying the effective ionic radii of the metal cations considered (circles drawn to scale relative to ionic radius of Li+). Right: Chemical structures of VIm, MMA, and the [Tf2N]- anion.
For systems containing VIm or MMA with Mn+(Tf2N-)n, not all salts could be dissolved in these monomers up to 1:1 monomer:salt ratios (and form liquids at room temperature) in the same way LiTf2N could be dissolved. Nontheless, the addition of these alkali and alkaline earth metal bistriflimides still produced enhancements in the polymerization of VIm and MMA. Figure 5 presents the behaviors of VIm and MMA with KTf2N added.
Figure 5: Comparison of monomer conversion with time for VIm (left) and MMA (right) systems with various ratios of monomer:KTf2N.
As can be seen in Figure 5, the addition of KTf2N to VIm and MMA results in improved monomer conversion and rate compared to the neat monomer, although the kinetics for MMA + KTf2N are slower than for MMA + LiTf2N (Figure 3).
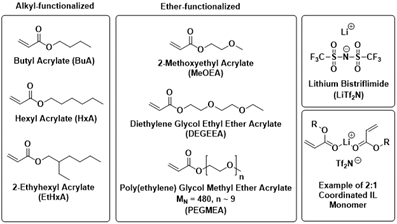
We have also extended our studies to acrylates with longer alkyl groups on the ester as well as analogous ether-based monomer species as shown in Figure 6.
Figure 6: Molecular structures of alkyl- (left) and ether- (center) functionalized monomers examined; LiTf2N (right, top); generic structure of 2:1 coordinated IL monomer (right, bottom).
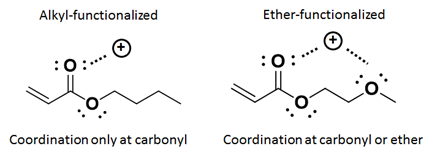
While the alkyl acrylate species can only experience Li+ coordination at the carbonyl, the ether-based acrylates can experience coordination at both the carbonyl and the ether as depicted in Figure 7.
Figure 7: Illustration of coordination sites in alkyl-functionalized acrylate monomers (left) and ether-functionalized monomers (right).
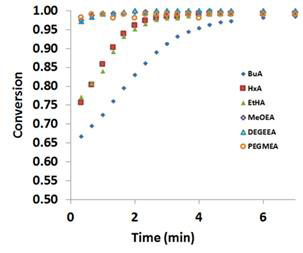
The ether-based monomers are thus expected to experience different behaviors in the presence of LiTf2N than alkyl-based acrylates. Ether-based acrylates have been shown to polymerize much more quickly than the alkyl-based monomers as shown in Figure 8.
Figure 8: Conversion with time for neat monomers (cf. Figure 6) without addition of LiTf2N.
The addition of LiTf2N had little impact on changing the polymerization kinetics of the ether-based monomers compared to the improvements achieved in alkyl-based monomers as summarized in Table 1.
Table 1: Improvement in conversion at 20 s of monomer-LiTf2N mixtures* relative to neat monomer samples.
Monomer |
Increase in Conversion at 20 s (%) |
BuA |
49.85 |
HxA |
32.14 |
EtHxA |
29.73 |
MeOEA |
2.62 |
DEGEEA |
2.92 |
PEGMEA |
1.91 |
*Since all salt-containing solutions displayed ~100% monomer conversion at 20 s, the % increase in conversion was found using a conversion of 1.00 for solutions containing LiTf2N.
From the polymerization data and corresponding FT-IR spectra, it can be concluded that Li+ coordinated to carbonyls in alkyl-functionalized acrylates is likely having an organizational effect in “structuring” these monomers, creating ionic “heads” and non- ionic tails. Since the acrylate functionalities is located near the ionic head, they will be in closer proximity and will polymerize more rapidly. However, when there is competition between acrylate and ether functional for the Li+ cation, such an improvement is not observed and the ether-based acrylates already polymerize rapidly without any added LiTf2N.
Our current work focuses on the study of cross-linked systems and how the presence of LiTf2N changes the morphology of the cross-linked polymer and its ability to swell in the presence of water and polar organic solvents.