Reports: DNI655535-DNI6: Influence of Low Temperature Chemistry and Chemical Classes on MILD Combustion of Large Hydrocarbon Fuels
David Blunck, PhD, Oregon State University
1. Introduction
Moderate or Intense Low-Oxygen Dilution (MILD) combustion has the potential to significantly reduce pollutant emissions from applications requiring burning large hydrocarbon (i.e., liquid) fuels. Prior fundamental research has mainly focused on MILD combustion of gaseous fuels. Consequently, it is not well understood how MILD combustion changes when different classes of large hydrocarbon fuels (e.g., aromatics, alkanes) are burned. With this motivation, the overall objectives of this research are as follows:
1) Discover how MILD combustion changes when different classes of large hydrocarbon fuels are burned; 2) Determine the effect of low temperature chemistry on the stability of MILD combustion burning these fuels; and 3) Discover the chemical kinetic pathways which are responsible for changes in MILD combustion when low temperature chemistry is significant.
The focus of this past year has been to (a) repeat previous experiments and verify that we obtain consistent results and (b) evaluate the MILD behavior of surrogate jet fuels. These focuses contribute to achieving objective 1.
2. Experimental and Computational Approaches
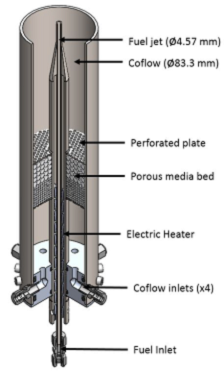
An experiment and apparatus were designed and used to help achieve objective 1 (during the first year of the project). MILD combustion was created using a jet in hot cross-flow burner (JHC), as shown in Figure 1.
Figure 1. Illustration of the jet in hot cross-flow burner.
The JHC burner consists of a central fuel jet surrounded by a coflow of hot combustion products. MILD or other high temperature conditions were created around the central fuel jet within about 10 cm of the burner exit.
liftoff height of the flame was measured using chemiluminescence of OH* emissions. Visible images of the reaction zone were not always evident, or did not always agree with the flame location found using chemiluminescence.
During this past year, liftoff heights were measured as the following parameters were varied: the coflow temperature, the coflow oxygen concentration, and the Reynolds number of the central jet. The Reynolds number was changed from near 4,000 to 10,000. Disagreements between prior and current data have been investigated.
To study the effects on a surrogate fuel, two fuels were tested. First, jet-A was combusted to determine a baseline. This fuel has been tested previously on this burner. Next, a surrogate fuel (POSF-12765) for jet fuel was burned.
3. Results and Discussion
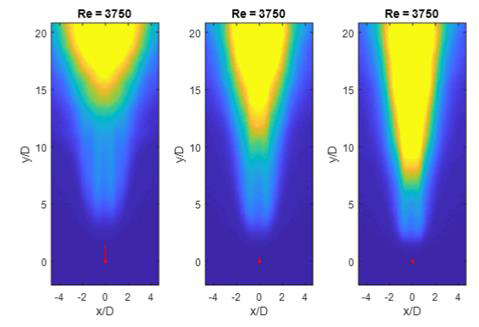
Chemiluminescence images of jet-A burning in coflows with 3% oxygen concentration are shown in Figure 2. The coflow temperature was varied between 1300, 1400, and 1500K.
Figure 2. Chemiluminescence from flames burning Jet-A as the coflow temperature was varied from 1300K to 1500K. The oxygen concentration of the coflow was kept constant at 3%. The red marks indicate the base of the flame.
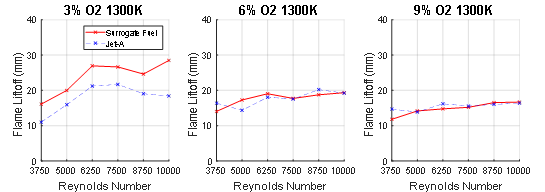
The liftoff height for flames burning the two fuels are reported in Figure 3 as the coflow oxygen concentration was varied from 3% to 9%. Three trends in the flame liftoff heights are evident.
Figure 3. Liftoff heights of the flame as the oxygen concentration of the coflow was varied from 3% to 9%. The temperature of the coflow was 1300K.
First, the flame liftoff height for both the surrogate and jet-A fuels decreased as the O2 concentration of the coflow increased. For the 6% and 9% O2 concentration conditions, both fuels exhibited lower liftoff heights than the 3% condition. This trend was observed for all Reynolds numbers. Hence, increases in the O2 concentration increases the laminar flame speed which tends to decrease the flame height. The second trend which is observed is that at the 3% O2 condition, the flame liftoff height increased for both fuels as their Reynolds number increased until a Reynolds number near 7500, after which the liftoff height tended to decrease. The decrease is attributed to an increase in CH2O production, which increases with flame stretch (i.e., greater turbulence). The increase in CH2O production as the Reynolds number increases has been observed with small hydrocarbon fuels using PLIF measurements. As the oxygen concentration increases, the influence of CH2O on liftoff height is reduced, and therefore the trend of decreasing liftoff height at higher Reynolds numbers is not replicated in the coflow conditions with reduced O2 concentration. The third trend noted is the liftoff height for the surrogate and jet-A fuel are similar for the conditions near 6% and 9% O2 concentration. This is expected, considering that these conditions are more like conventional combustion; the conditions at which surrogate fuels are evaluated (although at laminar conditions). In contrast, at 3% O2, the liftoff height of the surrogate fuel flame is higher than the jet-A fueled flame. It is plausible that the discrepancy is caused by changes in CH2O production because of the fuel chemistry.
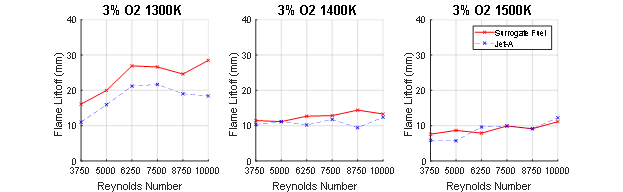
The temperature of the coflow was varied and the results plotted below in Figure 4.
Figure 4. Liftoff heights of the flame as the temperature of the coflow was varied from 1300K to 1500K. The oxygen concentration remained constant at 3%.
Significant changes in average liftoff height and fuel sensitivity occurred when changing the temperature of the coflow. The liftoff height of both fuels tends to decrease as the temperature of the coflow increases. At the lowest temperature, the liftoff heights are the highest, with the highest liftoffs occurring near Reynolds numbers of 6250 or 7500. As the flames become more turbulent and the turbulence intensity increases, flame stretch also increases, which can increase CH2O production. The higher turbulence intensity is also known to increase the production of CH2O. As the temperature of the coflow increases, the liftoff heights of both decrease. The higher temperature gas in the coflow increases the reactivity of the fuel, thereby lowering liftoff heights.
4. Future Work
Future work will consist of two primary tasks. First, identifying how the location of OH and CH2O within the reaction zone change for different fuel or coflow conditions. This will be achieved by applying planar laser induced fluorescence (PLIF). Second, elucidating the sensitivity of MILD combustion to low temperature chemistry. The burner may be modified to enable air dilution of the central fuel jet or injection of CH2O.