Reports: UR756381-UR7: Stereoregular Vinyl Polymers Prepared by Reversible-Deactivation Radical Polymerization
Eric S. Tillman, PhD, Santa Clara University
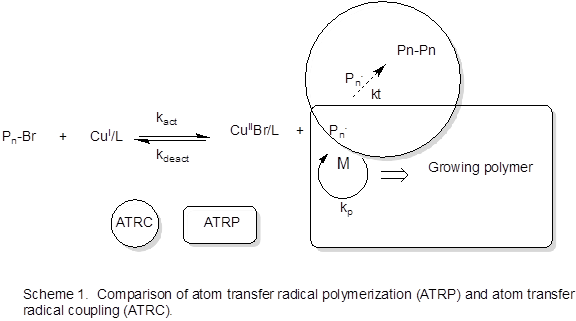
Atom transfer radical coupling (ATRC) and polymerization (ATRP) are compared in Scheme 1. Our group’s current thrust in this project supported by PRF has been to synthesize fluorinated vinyl aromatic polymers (such as poly(pentafluorostyrene), PPFS) and employ these end-brominated macromolecules in ATRC or radical trap-assisted ATRC reactions.
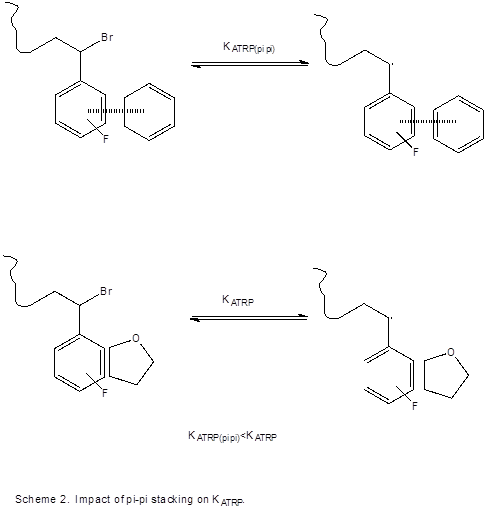
The impact of pi-pi stacking on post-polymerization transformations has not, to our knowledge, been stuided. Specifically, we have found that coupling of polymer chains occurs at a slower rate when the pendant group is involved in pi-pi stacking with an external agent, as visualized in Scheme 2. Our current hypothesis is the decreased activation of the C-Br bond in atom transfer reactions is responsible for the observed rate adjustments, regarding polymer end groups involved in pi-pi stacking when compared to those that are not. The position of KATRP directly affects the rate at which the coupling occurs, as the rate is directly tied to the concentration of polymer radical.
We have found that the addition of benzene or toluene results in lowered extents of coupling compared for PPFS compared to analogous trials performed in the absence of pi-pi stackers. This was somewhat unexpected, as donating electron density into the chain end via pi-pi stacking was hypothesized by us to stabilize the electron deficient, benzylic radical and result in faster rates of couplings.
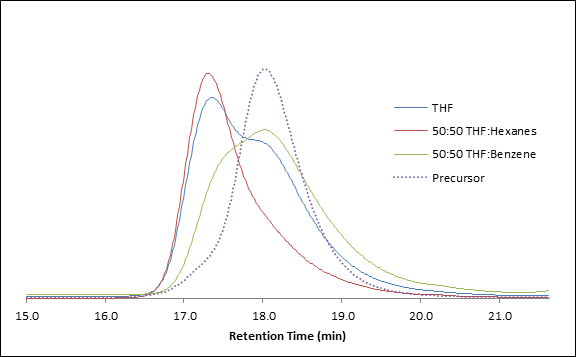
Pi-pi stacking aside, our results have also confirmed that polymer chains with fluorinated end groups undergo coupling at a match faster rate than their non-fluorinated analogs (at least in the case of styrenic polymers) when pi-pi stacking is not involved. This is an exciting find, as it has allowed us to perform coupling reactions at room temperature and achieve high yields of dimerized polymer chains. Figure 1 shows the GPC traces of a PPFS precursor (dotted) alongside that of the coupled product when performed in different solvents. The reaction is outlined in Scheme 3. The clear result is that benzene’s presence slows down the rate of the coupling reaction, as it pi-pi stacks with the chain end radical and decreases its stability. As a control, the same mixed solvents and additives were used in the coupling of non-aromatic and non pi-p stacking systems (we chose brominated poly(methylacrylate)), and the presence of the external aromatic face did not impact the rate of dimerization.
Figure 1. Brominated PPFS precursor (prepared by ATRP, dotted) along with results of RTA-ATRC when performed in: pure THF, THF/Hexanes, and THF/Benzene. Reaction conditions: 2-methyl-2-nitrosopropane (MNP) as the radical trap; room temperature for 60 min. [PPSF]:[MNP]:[Cu]:[CuBr]:[PMDETA] = 1;0.6:1:1:2.
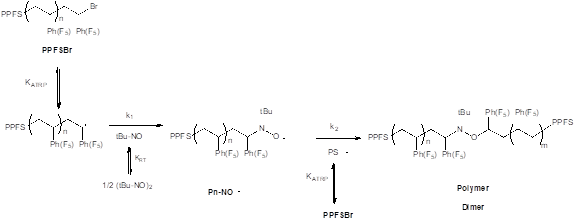
Scheme 3. Coupling of brominated PPFS using RTA-ATRC. Note the KATRP and the KRT are affected by solvent environment.
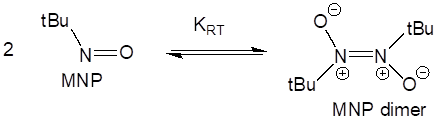
The improvement of THF/Hexanes when compared to pure THF is explained by the position of KRT, which is solvent-dependent. The more polar solvent THF favors the zwitterionic dimer, while non-polar solvents like hexanes and benzene shift the equilibrium further toward the active monomer. This can be seen is Scheme 4.
Scheme 4. The active monomer (left) and the inactive radical trap aggregate (right) are in equilibrium with each other. More polar solvents, like THF, push the equilibrium further towards the right.
The following current and future plans to extend this work are:
1) To place small segments of fluorinated chains at the end of other polymers, allowing the experimenter to couple stubborn or non-ATRC compatible polymer chains. This is tricky, because the rate of polymerization for pentafluorostyrene is very fast and therefore difficult to control. Thrust #3 below deals with this dilemma.
2) To translate these results into cyclizaton reactions, producing cyclic polymers in high yields even at at room temperature. These reactions will be carried out with PPFS homopolymers, or BAB triblocks with PPFS segments on the chain ends.
3) Use pi-pi stacking to decrease the rate of reactions that rely of the position of KATRP. For example, using pentafluorostyrene as a monomer in ATRP reactions results in a very fast polymerization. It is difficult to control the length of the chains, especially when placed on the end of other polymers (block copolymer synthesis). Could we use the addition of pi-pi stacking agents to suppress the rate?